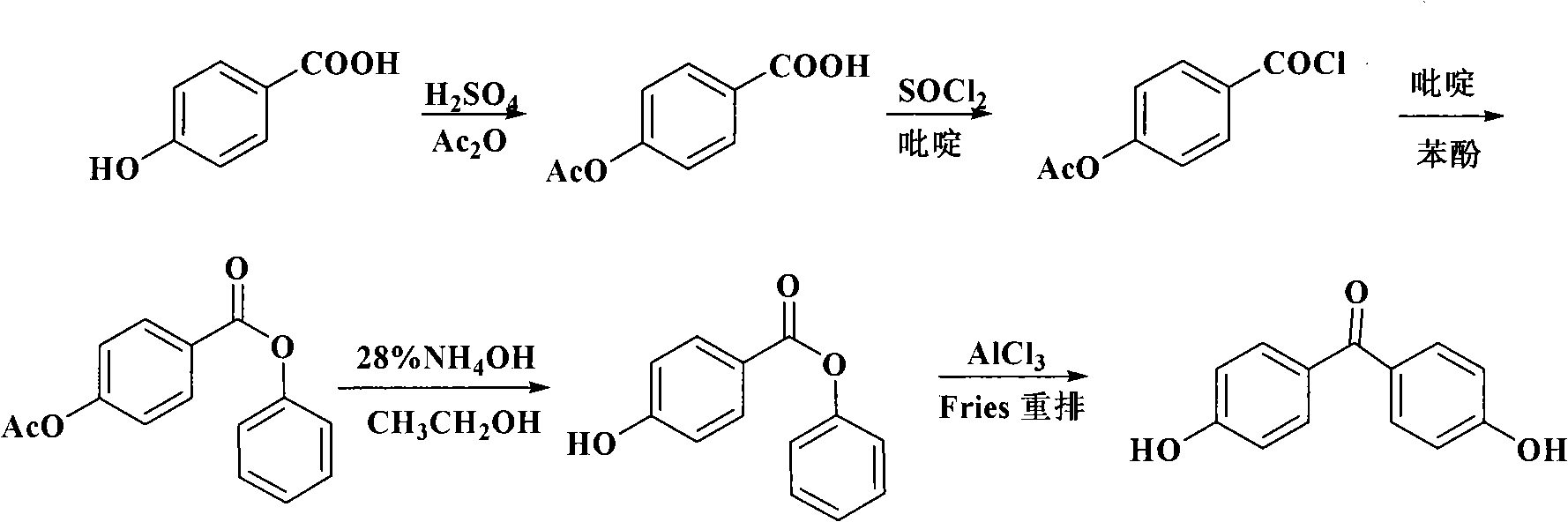
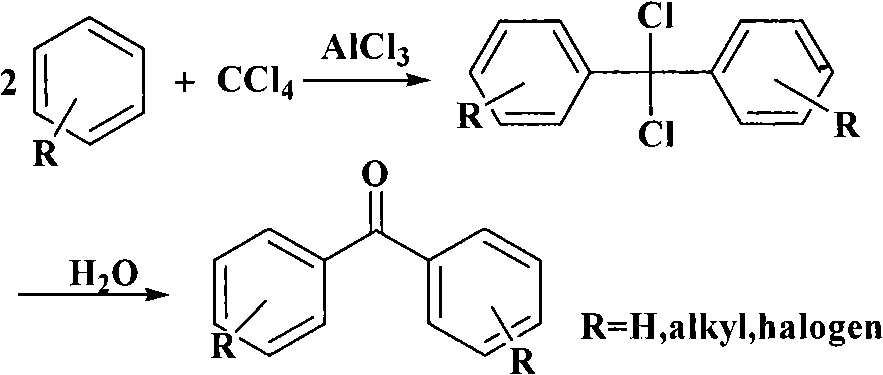
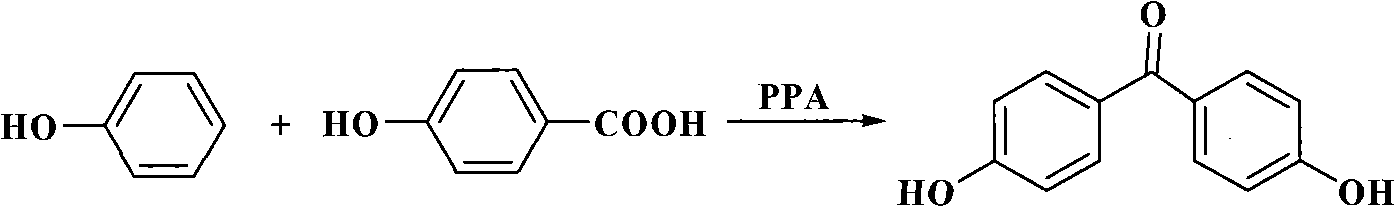Process for synthesizing 4,4'-dihydroxy diphenylketone
A technology of dihydroxybenzophenone and synthetic methods, applied in 4 fields, can solve problems such as environmental pollution, achieve the effects of reducing production costs, easy handling and storage, and realizing resource utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: the preparation of solid superacid catalyst
[0031] FeCl 3 ·6H 2 O10g was dissolved in 100mL distilled water, and 25% (w / w) ammonia water was added to adjust the pH to 8 to obtain ferric hydroxide sol, which was filtered, and the filter cake was washed with distilled water to remove Cl - , dried, placed in 0.25mol / L H 2 SO 4 Solution (15mL / g) was impregnated, then filtered, dried, and roasted at 500°C to obtain the solid superacid catalyst (SO 4 2- / Fe 2 o 3 ).
Embodiment 2
[0033] Take commercially available Fe(NO 3 ) 3 9H 2 O10g was dissolved in 100mL distilled water, and urea was added to adjust the pH to 9 to obtain ferric hydroxide sol, which was filtered, and the filter cake was washed with distilled water to remove NO 3 -, dried, placed in 0.50mol / L (NH 4 ) 2 SO 4 Solution (15mL / g) was impregnated, then filtered, dried, and roasted at 550°C to obtain the solid superacid catalyst (SO 4 2- / Fe 2 o 3 ).
Embodiment 3
[0035] commercially available anhydrous AlCl 3 Dissolve 5 g in 50 mL of distilled water, add 25% (w / w) ammonia water to adjust the pH to 8, obtain aluminum hydroxide sol, filter, and wash the filter cake with distilled water to remove Cl - , dried, placed in 2.50mol / L H 2 SO 4 Solution (15mL / g) was impregnated, then filtered, dried, and roasted at 600°C to obtain the solid superacid catalyst (SO 4 2- / Al 2 o 3 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com