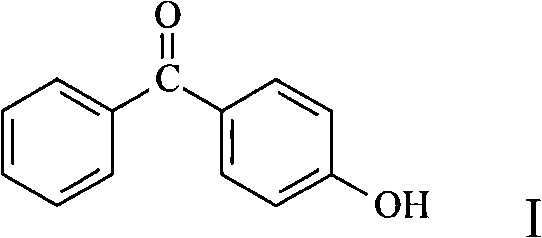
Preparation of 4-hydroxy benzophenone
A technology of hydroxybenzophenone and trihalomethylbenzene, which is applied in the field of preparation of 4-hydroxybenzophenone, can solve problems such as low atom utilization, unsuitability for large-scale commercial preparation, and complicated steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
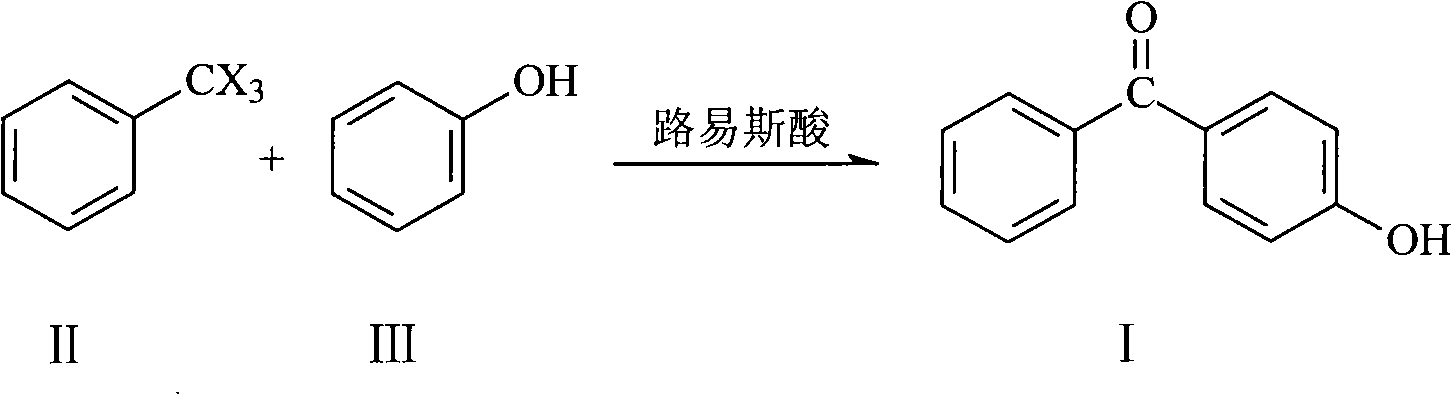
[0019] The method for preparing 4-hydroxybenzophenone according to the present invention includes the following steps:
[0020] Under the condition of 0℃~5℃, the 1,2-dichloroethane solution of catalyst (Lewis acid), trichloromethylbenzene and phenol were added to 1,2-dichloroethane in sequence. Among them, phenol and catalyst The molar ratio of phenol is 1:(1.1-1.5), and the molar ratio of phenol to trichloromethylbenzene is 1:(1.1-1.4). After feeding, react at 20°C~25°C (thin-plate chromatography "tracks" the reaction, and reacts until the raw material point disappears),
[0021] Under stirring conditions, the reaction liquid was poured into ice / water, and solid matter was precipitated. After filtration, the obtained solid matter was recrystallized and dried to become the target (4-hydroxybenzophenone). The filtrate can be distilled to recover the reaction flux (1,2-dichloroethane), which can be recycled.
[0022] By adopting the method for preparing 4-hydroxybenzophenone accordi...
Embodiment 1
[0025] Add 1,2-dichloroethane (80ml) and anhydrous AlCl to a 250ml three-necked flask 3 (20g, 0.15mol), cooled in an ice bath to 0℃-5℃, stirred for half an hour to make anhydrous AlCl 3 Disperse into fine particles, and then add trichloromethylbenzene (15.7ml, 0.11mol) dropwise. The addition is completed within 20 minutes, and then the reaction is incubated for 20 minutes, at which time the reaction solution turns orange-red. Phenol (9.41g, 0.1mol) was dissolved in 1,2-dichloroethane (20ml), the phenol solution was added dropwise at 0°C-5°C, and the drop was completed within 20 minutes. At this time, a large amount of HCl gas is generated, which is absorbed with dilute lye through the gas absorption device. At the later stage of the reaction, when the color of the reaction mixture turns dark purple, the temperature is raised to 20°C-25°C, and the reaction is carried out for 3 hours. After the completion of the reaction, the reaction solution was poured into ice / water with stirring...
Embodiment 2
[0027] Add 1,2-dichloroethane (80ml) and anhydrous AlCl to a 250ml three-necked flask 3 (14.69g, 0.11mol), cooled in an ice bath to 0℃-5℃, stirred for half an hour to make anhydrous AlCl 3 Disperse into fine particles, add trichloromethylbenzene (15.7ml, 0.11mol) dropwise, add it in 20 minutes, keep the reaction for 20 minutes, at this time the reaction solution turns orange-red. Phenol (9.41g, 0.1mol) was dissolved in 1,2-dichloroethane (20ml), the phenol solution was added dropwise at 0°C-5°C, and the drop was completed within 20 minutes. At this time, a large amount of HCl gas is generated, which is absorbed with dilute lye through the gas absorption device. At the later stage of the reaction, when the color of the reaction mixture turns dark purple, the temperature is raised to 20°C-25°C, and the reaction is carried out for 3 hours. After the completion of the reaction, the reaction solution was poured into ice / water with stirring, and allowed to stand at 10°C for 2 hours. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com