Preparation methods of 2-(trifluoromethyl)benzaldehyde and intermediate thereof
A technology of trifluoromethylbenzaldehyde and trifluoromethyldichlorotoluene is applied in the field of preparation of o-trifluoromethylbenzaldehyde and intermediates thereof, and can solve the problems of unsuitability for industrialized production, complicated operation and high cost, Achieve the effect of easy industrial production, simple process and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
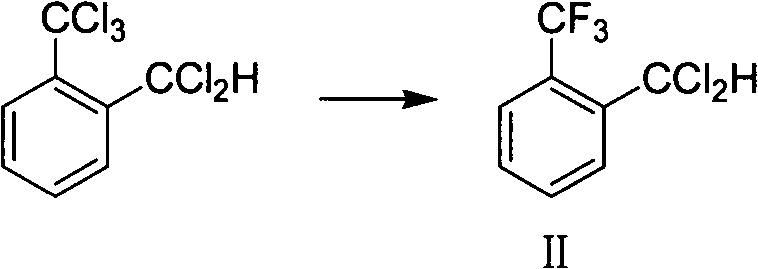
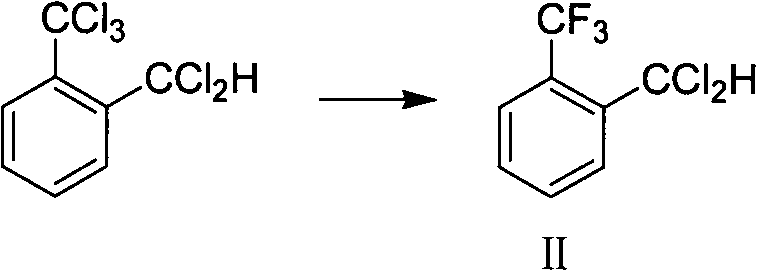
[0048] Put 278g (1.0mol) of crude o-trichloromethyldichlorotoluene and 2g of antimony trifluoride into the autoclave, cool down to 10°C, feed 80g (4.0mol) of hydrogen fluoride into the autoclave, heat up to 70°C, and keep the temperature at 70°C React between -90°C, control the pressure of the kettle not higher than 3.5MPa, after 4 hours of reaction, through gas chromatography analysis, o-trichloromethyldichlorotoluene <0.5%, release the pressure to normal pressure, purging with nitrogen for 1 hour, and cool down to At room temperature, add 15g of solid sodium carbonate, stir and filter with suction to obtain 224g of mother liquor, GC area: o-trifluoromethyldichlorotoluene 92%, vacuum distillation, collect 190.5g of fractions at 60-85℃ / -0.095MPa, GC Area: 97.2%, yield 93.5%.
Embodiment 2
[0050] Put 278g (1.0mol) of crude o-trichloromethyldichlorotoluene and 1.0g of antimony trichloride into the autoclave, cool down to 10°C, feed 70g (3.5mol) of hydrogen fluoride into the autoclave, heat up to 70°C, and keep warm at React between 70-90°C, control the pressure of the kettle not higher than 4.0MPa, after 4 hours of reaction, through gas chromatography analysis, o-trichloromethyldichlorotoluene <0.5%, release the pressure to normal pressure, purging with nitrogen for 1 hour, and cool down To room temperature, add 10.8g sodium carbonate solid, stir and filter with suction to obtain 223g mother liquor, GC area: 89.3% o-trifluoromethyldichlorotoluene, rectify under reduced pressure, collect 186.7g of fractions at 60-85℃ / -0.095MPa , GC area: 96.5%, yield 91.7%.
Embodiment 3
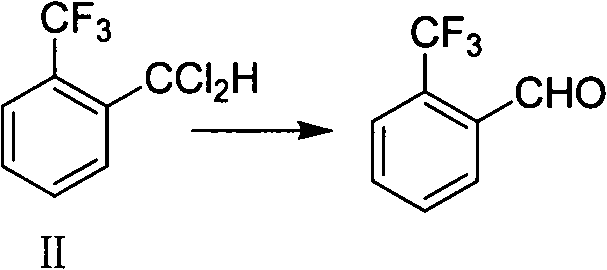
[0052] Put 114g (0.5mol) of o-trifluoromethyldichlorotoluene, 210g of 20% sodium hydroxide solution (1.05mol), 240g of acetic acid (4.0mol), and 0.1g of tetrabutylammonium bromide into the autoclave, and close the kettle. Raise the temperature to 160°C, the pressure is 0.45MPa, keep warm for reaction, after 3 hours of reaction, through gas chromatography analysis, o-trifluoromethyldichlorotoluene < 0.5%, cool down to room temperature, filter the reaction solution with suction, evaporate the acetic acid from the mother liquor under reduced pressure , add 240g of water, stir and stand to separate the layers, separate the organic layer, rectify under reduced pressure, collect the fraction at 70-75°C / -0.095MPa, and obtain 78.7g of o-trifluoromethylbenzaldehyde, GC area: 98.5%, Yield: 90.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com