Preparation method of 2,4-dichloro-5-fluorobenzoyl chloride
A technology of fluorobenzoyl chloride and dichlorofluorobenzene, which is applied in the field of organic synthesis to achieve breakthroughs in technical bottlenecks, high yields, and less pollutant emissions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: a kind of preparation method of 2,4-dichloro-5-fluorobenzoyl chloride, total yield is 89.3%.
[0042]
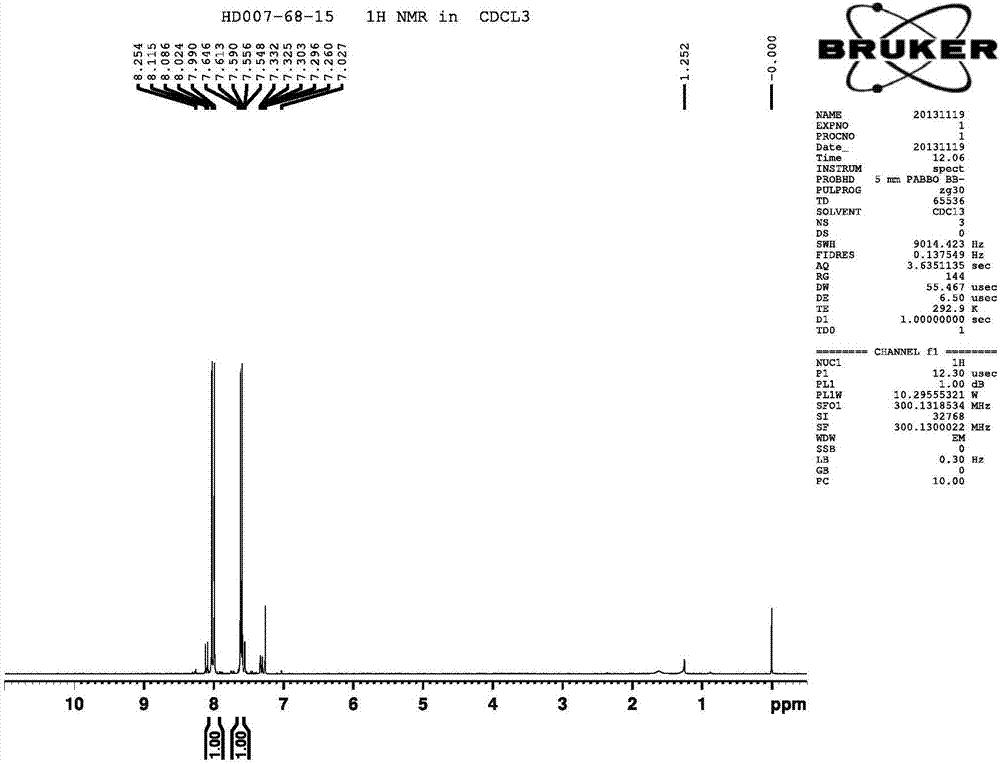
[0043] The raw material 2,4-dichlorofluorobenzene (100g, 0.606mol) was added into carbon tetrachloride (107.4g, 0.667mol), after stirring and dissolving, iron trichloride (1.52g, 9.3mmol) was added, and the temperature was raised to Maintained at 70°C for 2 hours, the liquid phase detection raw materials basically disappeared. Cool down to room temperature, add 2.5M HClaq (500ml) to quench the reaction, stir until clarified and separate layers, take the lower organic phase and distill under reduced pressure to obtain compound I (128.5g, 75.1%) and compound III (25.3g, 20.3%).
[0044] 1 H NMR (CDCl 3 300MHz): δ7.548-7.646(d,1H),8.024-8.115(d,1H).
[0045]
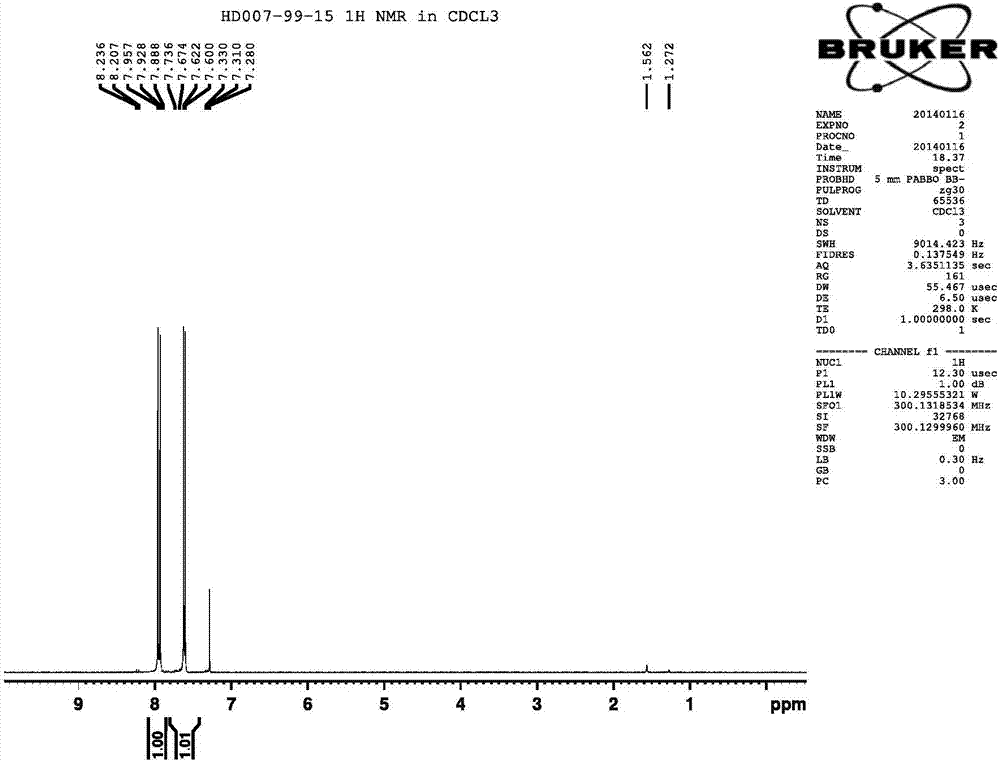
[0046]Add anhydrous ferric chloride (1g) to compound I (20g, 70.9mmol), heat to 145°C, slowly add water (1.27g, 70.9mmol) dropwise, and continue stirring for 30 minutes after adding, the ga...
Embodiment 2
[0056] Embodiment 2: a kind of preparation method of 2,4-dichloro-5-fluorobenzoyl chloride, total yield is 92.1%
[0057] Step (1): The steps are the same as in Example 1, the difference lies in step (1), the step (1) of this example is to add the raw material 2,4-dichlorofluorobenzene (10g, 60.6mmol) to four After stirring and dissolving in carbon chloride (9.27g, 57.6mmol), chlorine trichloride (0.124g, 0.93mmol) was added, the temperature was raised to 40°C and maintained for 2 hours, and the liquid phase detection raw materials basically disappeared. Cool down to room temperature, add 2.5M HClaq (50ml) to quench the reaction, stir until clarified, separate layers, take the lower organic phase and distill under reduced pressure to obtain compound I (13.1g, 76.6%) and compound III (2.8g, 22.6%)
[0058] Step (2): The procedure is the same as in Example 1, adding anhydrous ferric chloride (2g) to compound I (40g, 141.8mmol), and after heating to 140°C, slowly add water (2.54...
Embodiment 3
[0062] Embodiment 3: A kind of preparation method of 2,4-dichloro-5-fluorobenzoyl chloride, the total yield is 88.5%
[0063] Step (1): Same as Example 1, add the raw material 2,4-dichlorofluorobenzene (100g, 0.606mol) into carbon tetrachloride (107.4g, 0.667mol), stir and dissolve, then add trichloride Iron (1.52g, 9.3mmol), the temperature was raised to 70°C and maintained for 2 hours, and the raw material basically disappeared in the liquid phase detection. Cool down to room temperature, add 2.5M HClaq (500ml) to quench the reaction, stir until clarified, then separate layers, take the lower organic phase and distill under reduced pressure to obtain compound I (128.5g, 75.1%) and compound III (25.3g, 20.3%).
[0064] Step (2): same as Example 1, the difference lies in step (2), the step (2) of the present embodiment joins anhydrous ferric chloride (3g) in compound I (30g, 106.3mmol) After heating to 145°C, water (1.52g, 101.0mmol) was slowly added dropwise, stirring was c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com