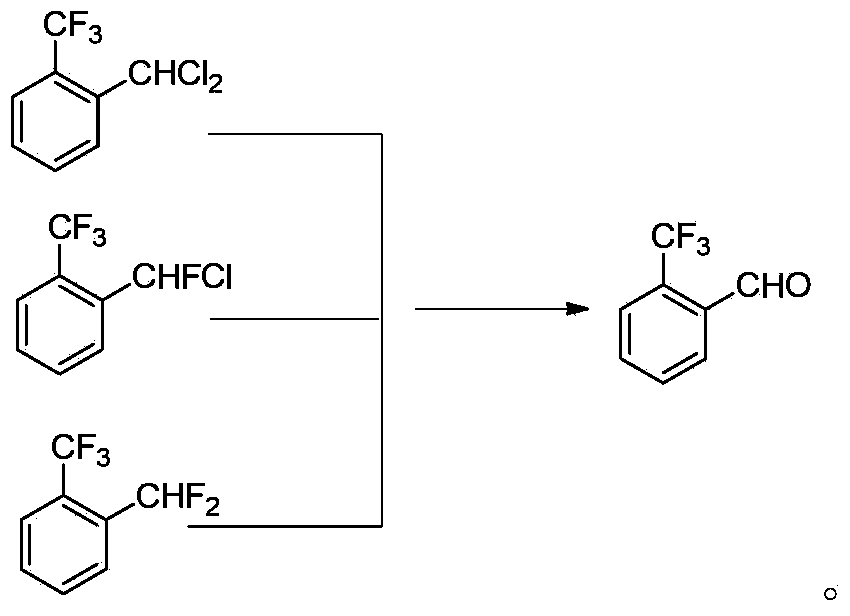
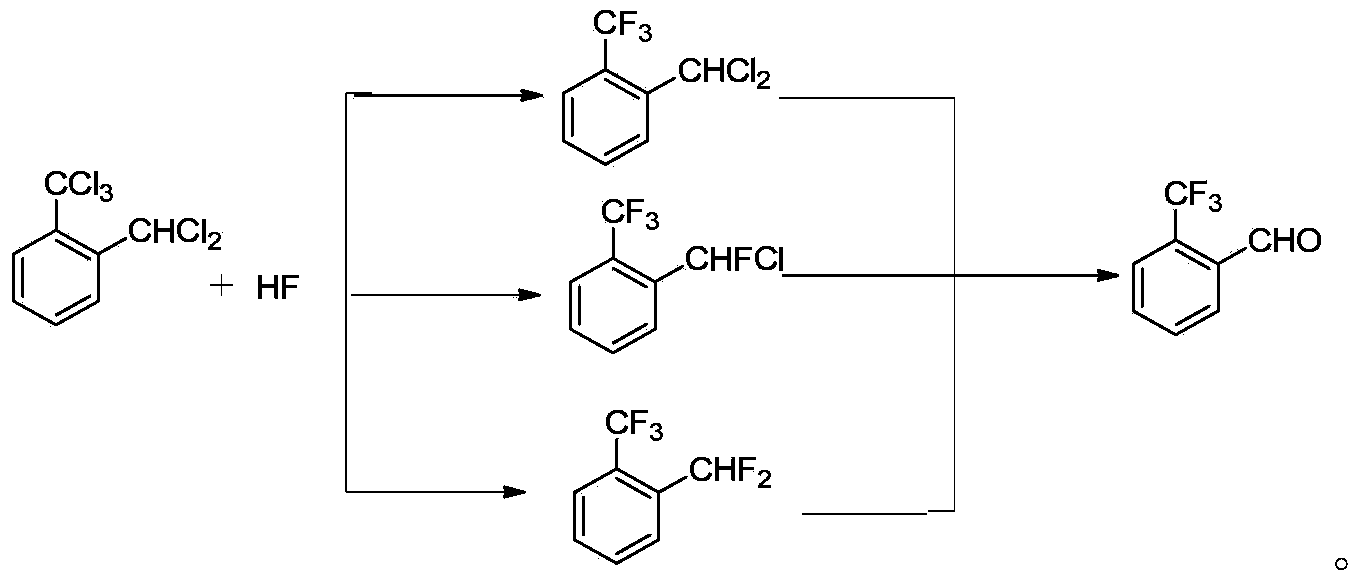
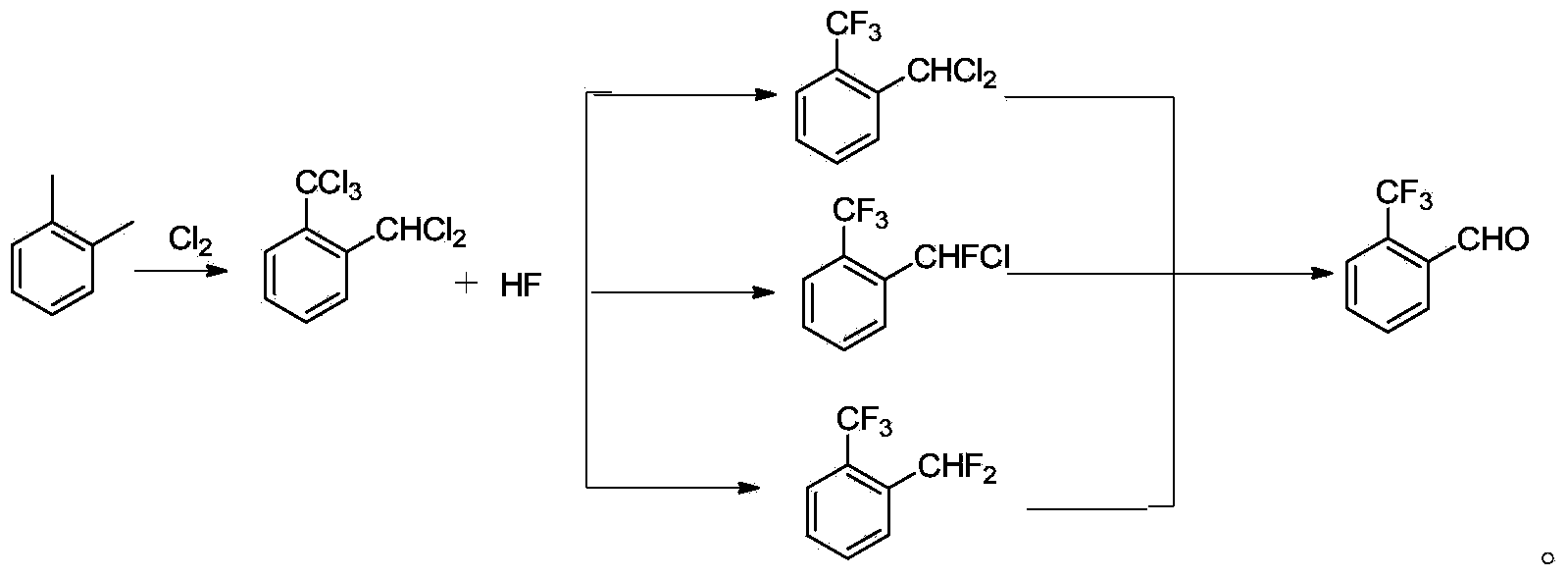
Method for preparing o-trifluoromethyl benzaldehyde
A technology of trifluoromethylbenzaldehyde and trifluoromethyldichloromethylbenzene, which is applied in the field of preparation of o-trifluoromethylbenzaldehyde, can solve the problems of high production cost, high energy consumption, cumbersome operation, etc., and achieve The effects of less waste, low cost, and easy industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Chlorination reaction
[0038] In the photochlorination tower of 1500L, drop into 650kg o-xylene, 5kg azobisisobutyronitrile, open the tower jacket steam to raise the temperature, open the light source (4 ultraviolet lamps of 250w), open the tail gas system, slightly negative pressure (- 0.01Mpa) operation, when the temperature in the tower reaches 90-110°C, start to feed chlorine gas, the amount of chlorine flow is 40-60kg / h, fast first and then slow, keep the temperature in the tower at 110-130°C in the later stage, and pass through gas chromatography Analysis, ortho-dichloromethyldichlorotoluene (GC%)95% is the reaction end point, after passing through, stop logical chlorine, and chlorination The reaction solution was transferred to a transfer tank, purged with nitrogen for 0.5 h, and kept at 70°C for use (melting point about 53-54°C).
[0039] Fluorination reaction
[0040] 2300kg of chlorinated material (o-trichloromethyldichloromethylbenzene, preparation method ...
Embodiment 2
[0041] Embodiment 2 fluorination reaction
[0042] 2300kg of chlorinated material (o-trichloromethyldichloromethylbenzene, the preparation method is the same as the chlorination reaction in Example 1) and 1kg of antimony trifluoride are dropped into the fluorination kettle, and the still condenser refrigerant (cold brine temperature- 15~20℃), open the kettle jacket and cool the refrigerant down to 10℃, press 0.1kg nitrogen gas from the HF storage tank into 350kg HF to the HF transfer tank, then press 400kg recovered HF from the HF recovery tank to the transfer tank, and then release it by gravity into the fluorination kettle. Close the relevant valves, open the jacket steam of the fluorination kettle to slowly heat up, and control the reaction temperature at 70-90°C. When the pressure of the kettle reaches 2.5MPa, open the vent valve to slowly discharge the pressure. After decompression, the pressure does not rise any more, keep the temperature at 90-95°C, and after 4 hours o...
Embodiment 4
[0049] Hydrolysis reaction
[0050] Put 1500kg of the fluorinated crude product obtained in Example 1 into the hydrolysis kettle, add 7.5kg of ferric chloride, open the tail gas absorption system, open the jacket steam to raise the temperature, when the temperature reaches 100°C, start to add 105kg of water dropwise, and keep the reaction temperature at 100°C Between ~ 110°C, after the addition of water dropwise, keep warm for 2 ~ 3h, take a sample and analyze it with gas chromatography, the o-trifluoromethyl benzaldehyde content (GC) > 95%, then transfer the material to the rectification kettle, open the vacuum system (- 0.095Mpa), after vacuum stabilization, open the jacket steam, heat up rectification, collect the cut of 70~75 ℃ / -0.095MPa, obtain 965kg o-trifluoromethylbenzaldehyde, content (GC%)>99.0%. The fraction before rectification (containing a small amount of raw material) and the fraction after rectification (containing a small amount of raw material) are applied me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com