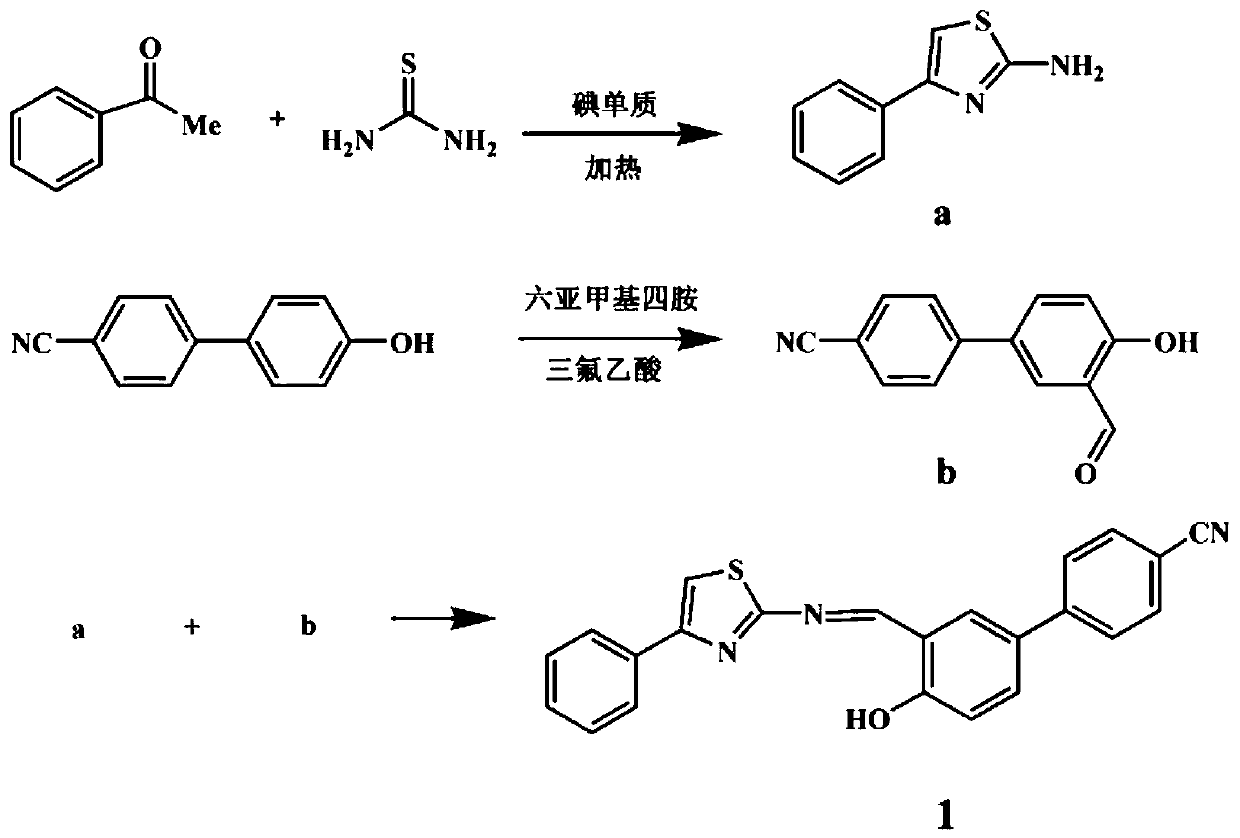
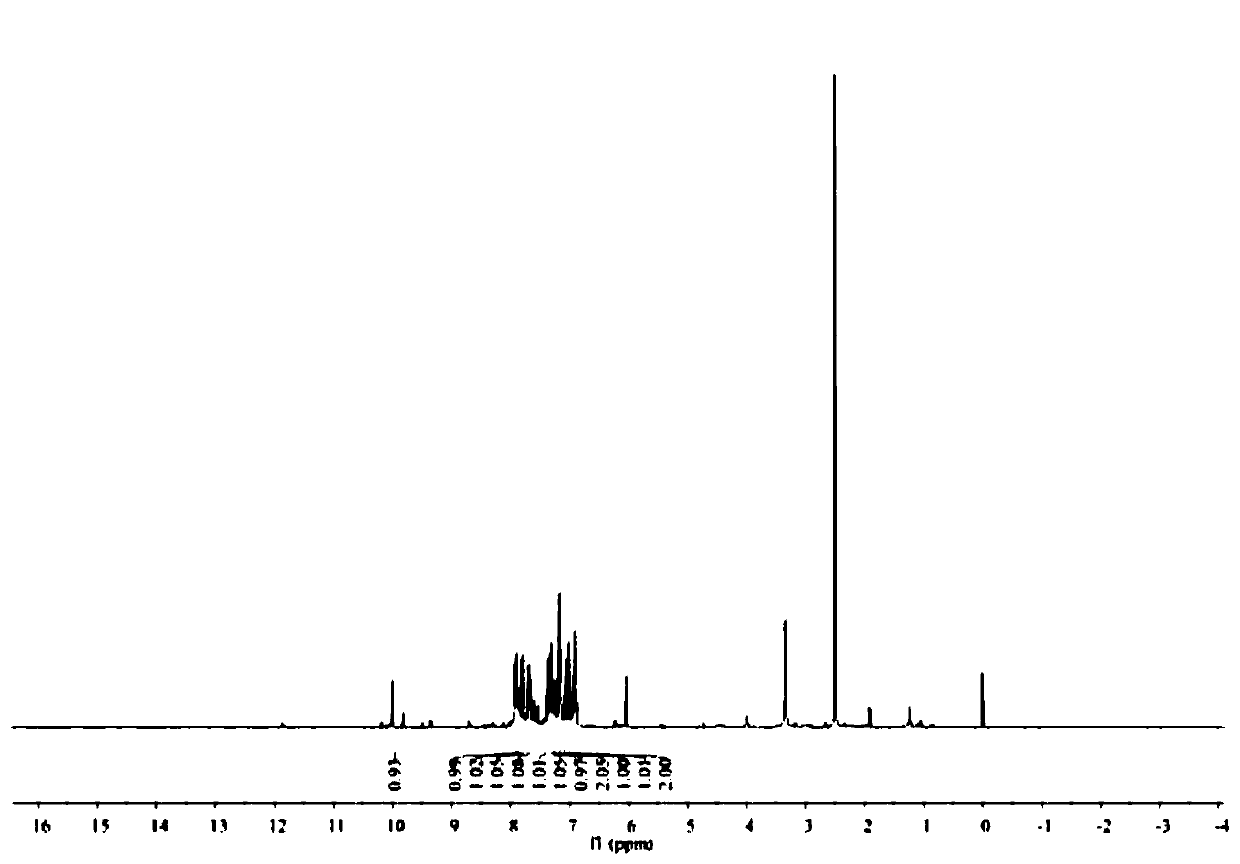
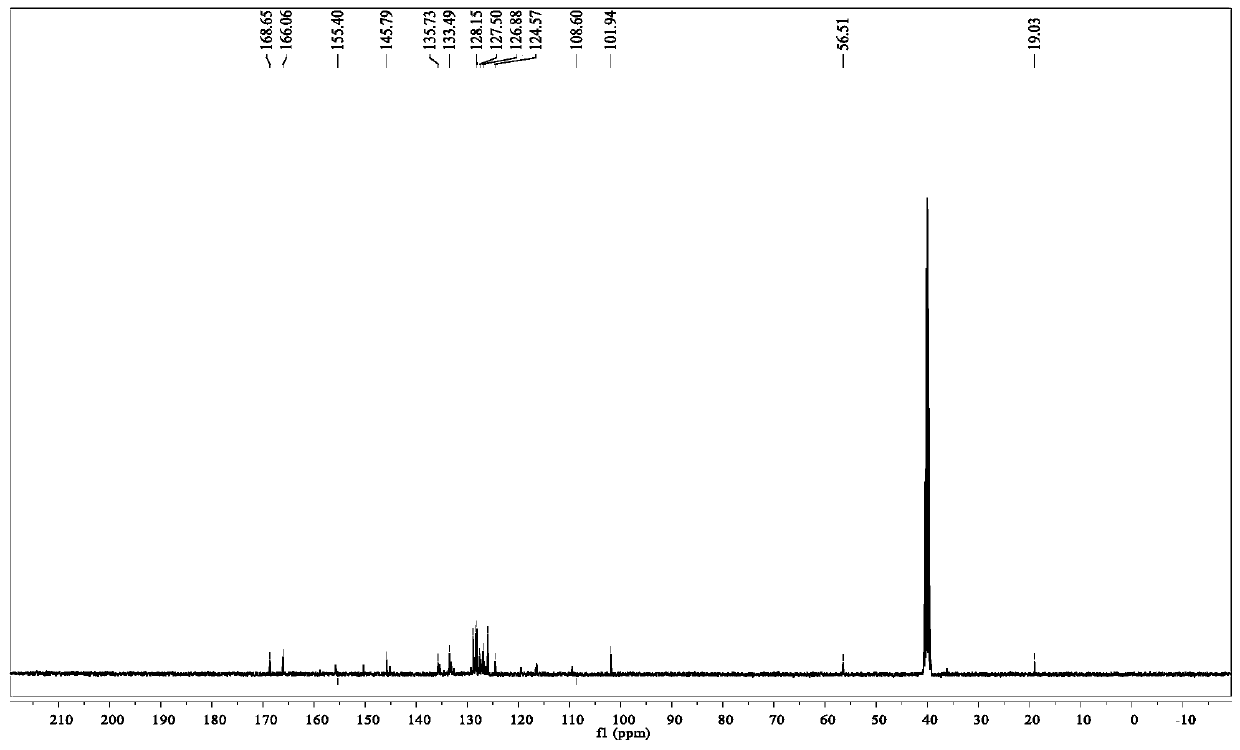
A fluorescent sensing material based on phenylthiazole and p-cyanobiphenol, its preparation method and application
A cyanobiphenol and phenylthiazole technology is applied in the field of chemical fluorescence sensing materials, which can solve the problems of inability to realize Fe3+ detection in biological cells, excessive introduction of organic solvents, complicated synthesis process, etc., and achieve simple and easy post-processing and purification. easy to control, the synthesis conditions are easy to control, and the synthesis conditions are mild
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] S1. Preparation of 2-amino-4-phenylthiazole: put 2.05g of acetophenone and 2.13g of thiourea into a round-bottomed flask, add 3.80g of iodine element, seal and react in an oil bath at 40°C for 24h, and react After the end, add hot water, stir and heat until it becomes a uniform solution system, filter, cool the filtrate to room temperature, then add ammonia water to adjust the pH to weak alkaline (pH=8), the product is precipitated and recrystallized in ethanol for purification.
[0040] S2. Preparation of 3-formyl-4-hydroxybiphenylcyanide: 0.5g of p-cyanobiphenol and 2.15g of hexamethylenetetramine were dissolved in 40mL of trifluoroacetic acid and placed in a round bottom flask at 100°C for 4 h under reflux. After the reaction, the reaction solution was cooled to room temperature, acidified by adding 1.0 M HCl (100 mL), and then extracted with dichloromethane. The collected organic layer was washed three times with water, dried over anhydrous magnesium sulfate, filter...
Embodiment 2
[0043] S1. Preparation of 2-amino-4-phenylthiazole: put 2.74g of acetophenone and 2.84g of thiourea into a round-bottomed flask, add 5.08g of iodine element, seal and react in an oil bath at 50°C for 27h, and react After the end, add hot water, stir and heat until it becomes a uniform solution system, filter, cool the filtrate to room temperature, then add ammonia water to adjust the pH to weak alkaline (pH=8), the product is precipitated and recrystallized in ethanol for purification.
[0044] S2. Preparation of 3-formyl-4-hydroxybiphenylcyanide: 1.0 g of p-cyanobiphenol and 5.30 g of hexamethylenetetramine were dissolved in 60 mL of trifluoroacetic acid and placed in a round bottom flask at 110°C for 6 h at reflux. After the reaction, the reaction solution was cooled to room temperature, acidified by adding 1.0 M HCl (100 mL), and then extracted with dichloromethane. The collected organic layer was washed three times with water, dried over anhydrous magnesium sulfate, filter...
Embodiment 3
[0047] S1. Preparation of 2-amino-4-phenylthiazole: put 3.42g of acetophenone and 3.55g of thiourea into a round-bottomed flask, add 6.35g of iodine element, seal and react in an oil bath at 60°C for 30h. After the reaction, add hot water, stir and heat until it becomes a uniform solution system, filter, cool the filtrate to room temperature, then add ammonia water to adjust the pH to weak alkaline (pH=8), the product is precipitated and recrystallized in ethanol for purification.
[0048] S2. Preparation of 3-formyl-4-hydroxybiphenylcyanide: 1.5g of p-cyanobiphenol and 6.45g of hexamethylenetetramine were dissolved in 80mL of trifluoroacetic acid and placed in a round bottom flask at 120°C for 8 h at reflux . After the reaction, the reaction solution was cooled to room temperature, acidified by adding 1.0 M HCl (100 mL), and then extracted with dichloromethane. The collected organic layer was washed three times with water, dried over anhydrous magnesium sulfate, filtered, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com