Preparation method and application of naphthylamine compounds and salts thereof
A compound, a technology of naphthylamine, which is applied in the field of preparation of naphthylamine compounds and their salts, can solve the problems of many nitro-reducing impurities, limited species expansion, poor solubility, etc., and achieves low-cost raw materials, inhibition of signal activation, and short steps. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A kind of preparation method of naphthylamine compound, synthetic route is as follows:
[0035]
[0036] (1) Preparation of compound b
[0037]Compound a (1.0 g, 8.19 mmol, 1.0 eq) and 1,2-dibromoethane (3.85 g, 20.47 mmol, 2.50 eq) were dissolved in 30 ml of anhydrous acetonitrile, and potassium carbonate (1.36 g, 9.83 mmol, 1.2 eq), potassium iodide (13.59 mg, 81.88 μmol, 0.01 eq). Raise the temperature to reflux for 16 hours, TLC monitors the completion of the reaction, filter with diatomaceous earth, spin dry, and purify by column chromatography (volume ratio, ethyl acetate:petroleum ether=15~5:1) to obtain 1.50 g of compound b as a white solid , yield 79.8%.
[0038] (2) Preparation of compound c
[0039] Compound b (1.0 g, 4.37 mmol, 1.0 eq) was dissolved in 40 mL of anhydrous tetrahydrofuran, sodium borohydride (198 mg, 5.24 mmol, 1.2 eq) was added at room temperature, and reacted at room temperature for 1 hour. TLC (petroleum ether: ethyl acetate = 3:1, R...
Embodiment 2
[0046] Embodiment 2: the preparation of compound h
[0047]
[0048] Dissolve compound g (100 mg, 265 μmol, 1.0 eq) in 10 ml of anhydrous methanol, add 37wt% concentrated hydrochloric acid (52.3 mg, 531 umol, 2.0 eq) at room temperature, continue the reaction at room temperature for 2 hours, and spin the solution directly Dry to obtain compound h, brown solid, 119 mg, yield 100%.
Embodiment 3
[0049] Embodiment 3: the preparation of compound i
[0050] Dissolve compound g (100 mg, 265 μmol, 1.0 eq) in 10 ml of anhydrous methanol, add 80% phosphoric acid aqueous solution (52 mg, 531 umol, 2.0 eq) at room temperature, react at 50°C for 12 hours, and spin the solution directly Dry to obtain compound i, a brown-black solid, 152 mg, yield 100%.
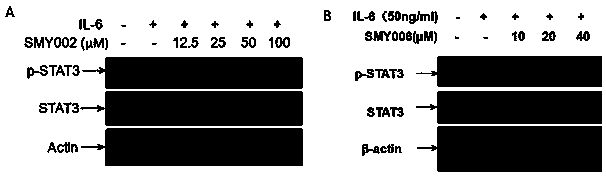
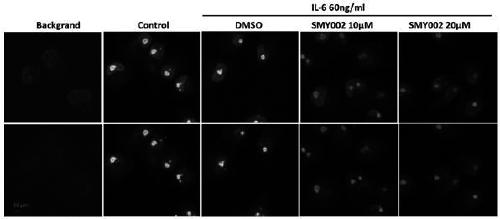
[0051] 1. Targeted inhibition of STAT3
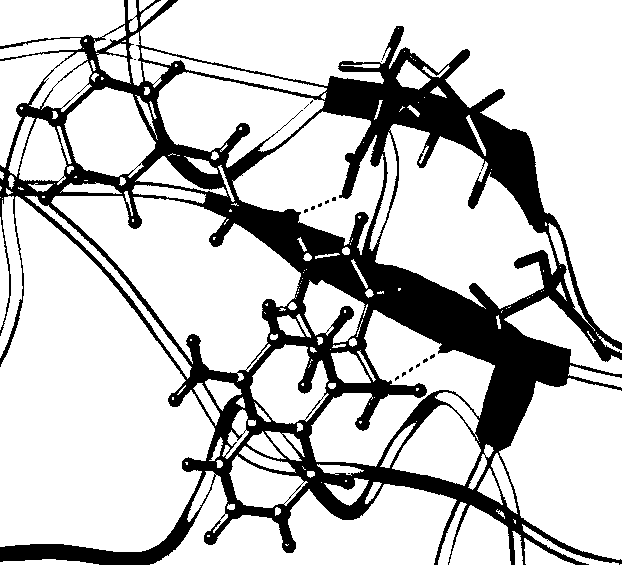
[0052] 1. Computer virtual analysis of the interaction between naphthylamino group and SH2 domain of STAT3 protein
[0053] All computer coordination simulation (docking) experiments were completed on the operating platform of sybyl X2.1.1, and the computer coordination simulation (docking) tool used was SUEFLEX DOCK. According to the selected sites (mainly including the phosphorylated tyrosine interaction sites of STAT3SH2 domain lysine (LYS) 591, arginine (ARG) 595 and arginine (ARG) 609 and hydrophobic interaction sites Glutamic acid (GLU) 638 was calculated, and the SH2 domain of S...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com