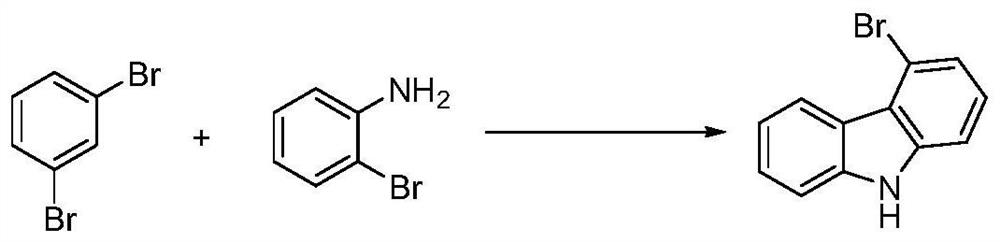
A kind of preparation method of 4-bromocarbazole
A technology of bromocarbazole and o-bromoaniline, applied in the field of chemistry, can solve problems such as unfavorable mass production, high cost, outstanding environmental protection problems, etc., and achieve the effects of simple structure, low production cost and good product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042]Under nitrogen protection, add 357.6g m-dibromobenzene (99%, 1.5mol), 26.1g o-bromoaniline (99%, 0.15mol), 0.35g three (dibenzylidene acetone) dipalladium ( 99%, 0.375mmol), 0.76g10% tri-tert-butylphosphine pentane solution (10%in Pentane, 0.375mmol), 61.2g triethylamine (99%, 0.6mol), after feeding, the temperature was raised to 150°C and stirred The rotation speed was 600rpm, and the heat preservation reaction was carried out for 24hr. After the reaction was completed, the unreacted m-dibromobenzene was recovered by distillation under reduced pressure, and water and ethyl acetate were added to the residue for extraction. Azole, content 99.5%, yield 89.3%.
Embodiment 2
[0044] Under nitrogen protection, add 357.6g m-dibromobenzene (99%, 1.5mol), 26.1g o-bromoaniline (99%, 0.15mol), 0.084g palladium acetate (99%, 0.375mmol), 0.18g in a 500mL reaction flask g 2-dicyclohexylphosphine-2,4,6-triisopropylbiphenyl (99%, 0.375mmol), 58.2g sodium tert-butoxide (99%, 0.6mol), after feeding, the temperature was raised to 130°C, The stirring speed was 600rpm, and the heat preservation reaction was carried out for 24 hours. After the reaction, the unreacted m-dibromobenzene was recovered by distillation under reduced pressure. Water and ethyl acetate were added to the residue for extraction. Carbazole, content 99.6%, yield 92.5%.
Embodiment 3
[0046] Under nitrogen protection, add 715.2g m-dibromobenzene (99%, 3mol), 26.1g o-bromoaniline (99%, 0.15mol), 0.17g palladium acetate (99%, 0.75mmol), 0.27g in 1L reaction flask n-Butylbis(1-adamantyl)phosphine (99%, 0.75mmol), 67.9g potassium tert-butoxide (99%, 0.6mol), after feeding, the temperature was raised to 110°C, the stirring speed was 600rpm, and the reaction was kept for 48hr. After the reaction, the unreacted m-dibromobenzene was recovered by distillation under reduced pressure, water and ethyl acetate were added to the residue for extraction, the organic layer was desolvated to obtain the crude product, and crystallized with ethanol to obtain 30.0 g of 4-bromocarbazole, the content of which was 99.1%. The rate is 80.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com