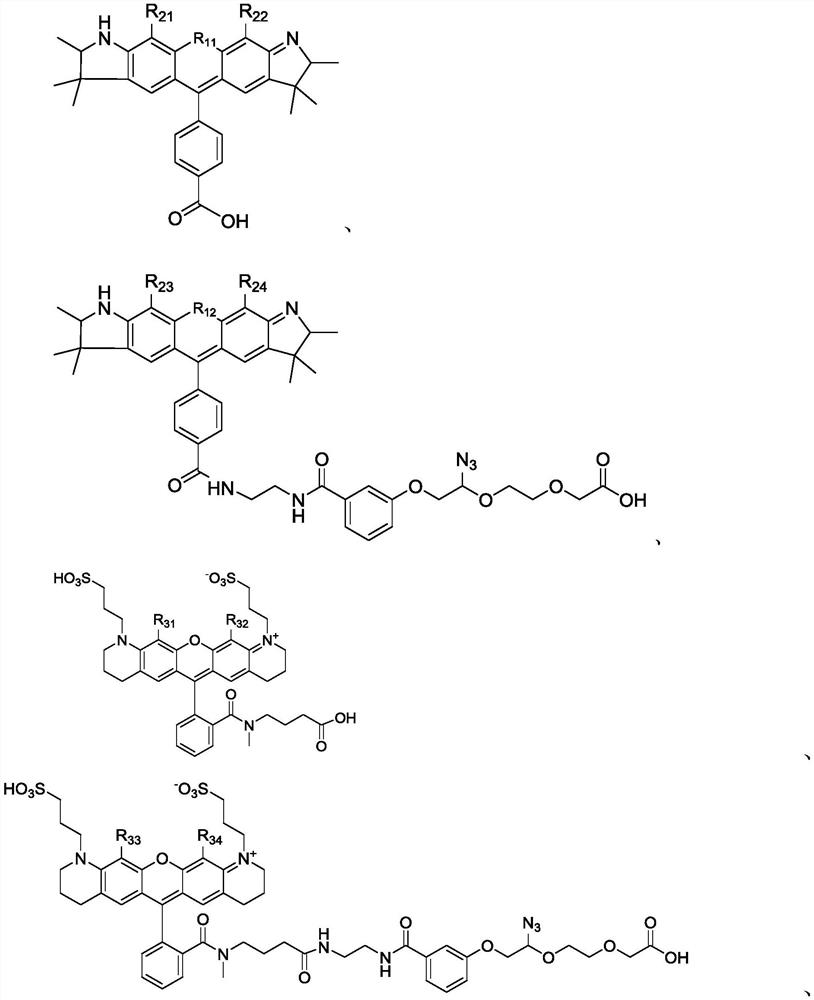
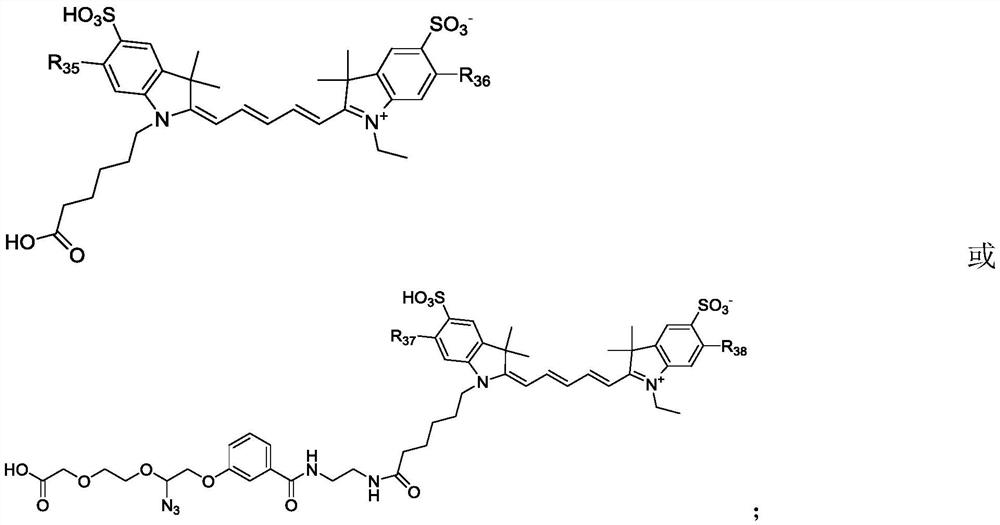
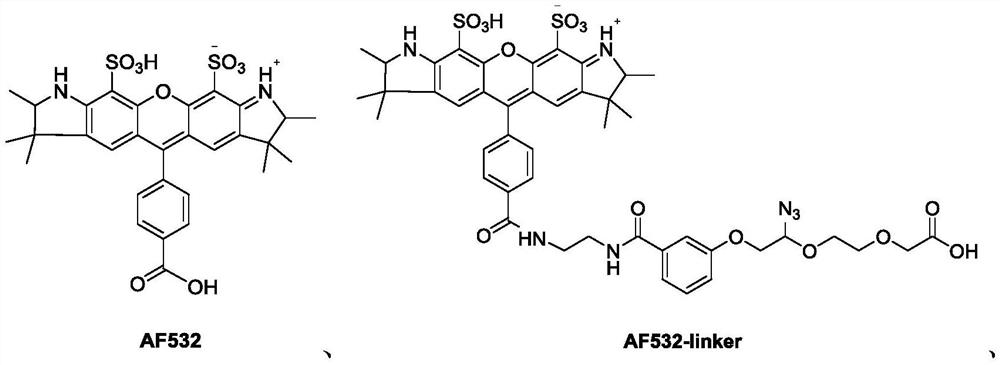
Succinimide esters and methods of making, treating and detecting same
A technology of succinimide ester and triethylamine, which is applied in measurement devices, organic chemistry, instruments, etc., can solve the problems of inaccurate detection methods, low yield and purity of succinimide ester, and solve unstable problems. The effect of improving the stability, improving the purity and improving the reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0150]
[0151] Synthesis of AF532-NHS:
[0152] Compound AF532 (1000mg, 1.60mmol, 1eq), TEA (1618mg, 16.02mmol, 10eq) were added to a 50mL three-necked flask, dissolved in DMF (10mL), nitrogen was replaced three times, and the reaction system was cooled to -20 using an ice-salt bath. Below ~-10°C, TSTU (1442 mg, 4.79 mmol, 3 eq) was added in five or more times, each addition of about 300 mg, with an interval of 5 minutes. Under the protection of nitrogen, the condensation reaction was carried out at a temperature below -10 °C for 1 h.
[0153] Postprocessing:
[0154] The reaction was added dropwise to 150 mL of dry dichloromethane (1 mL of acetic acid was added to 150 mL of dichloromethane), the pH of the resulting solution was 6-7, and a small amount of solid was precipitated, then 50 mL of dry PE was added, and it was allowed to stand for 2h. A large amount of powdery solid was precipitated, filtered, and the filter cake was evacuated to constant weight at 30° C. and ...
Embodiment 2
[0168] Synthesis method of AF532-linker:
[0169]
[0170] A 250mL two-necked flask was connected with a tee with a nitrogen balloon, and AF532-NHS (9.60g, 13.275mmol) and anhydrous DMF (96mL) were added to the two-necked flask to completely dissolve into an orange solution, and then DIPEA (5.15g, 39.825mmol) was added. ). Take another 50mL single-neck bottle in advance to weigh Linker (9.75g, 26.550mmol), add anhydrous DMF (48mL), and dissolve it into a colorless transparent liquid. Then, it was added to the above reaction system at one time, nitrogen was replaced three times, and the reaction was stirred at room temperature for 1 h. The reaction solution was poured into 1 L of ethyl acetate, and a solid was precipitated, which was filtered to obtain 14 g of a reddish-brown solid.
[0171] The mass spectral data are as follows: (positive ion mode)
[0172] AF532-linker: 796.3.
[0173]
[0174] Synthesis of AF532-linker-NHS:
[0175] Compound AF532-linker (1400mg, ...
Embodiment 3
[0191]
[0192] Synthesis of ROX C4-NHS:
[0193] Compound ROX C4 (1000mg, 1.33mmol, 1eq), TEA (1343mg, 13.3mmol, 10eq) were added to a 50mL three-necked flask. After dissolving with DMF (10mL), nitrogen was replaced three times, and the reaction system was cooled to - Below 20~-10 ℃, TSTU (1464mg, 3.99mmol, 3eq) was added in five or more times, about 300mg was added each time, and each time was 5 minutes apart. Under nitrogen protection, the condensation reaction was carried out at a temperature below -10 °C for 1 h.
[0194] Postprocessing:
[0195] The reaction was added dropwise to 150 mL of dry dichloromethane (1 mL of acetic acid was added to 150 mL of dichloromethane), the pH of the resulting solution was 6-7, and a small amount of solid was precipitated, then 50 mL of dry PE was added, and it was allowed to stand for 2h. A large amount of powdery solid was precipitated, filtered, and the filter cake was evacuated to constant weight at 30° C. and 1 mbar to obtain a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| percent by volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com