Triazine compound and preparation method thereof
A compound and triazine technology, applied in the field of organic chemical synthesis, can solve the problems of complex reaction process, waste of manpower and material resources, etc., and achieve the effects of simple reaction operation, less side reactions and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
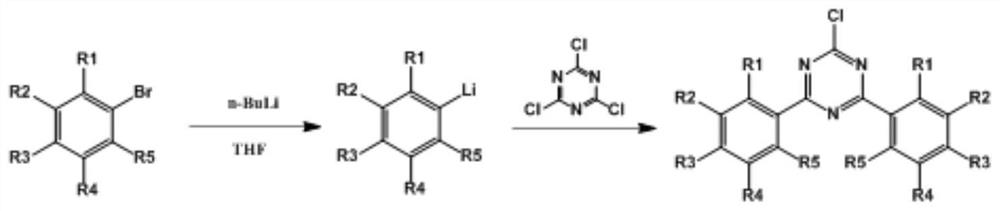
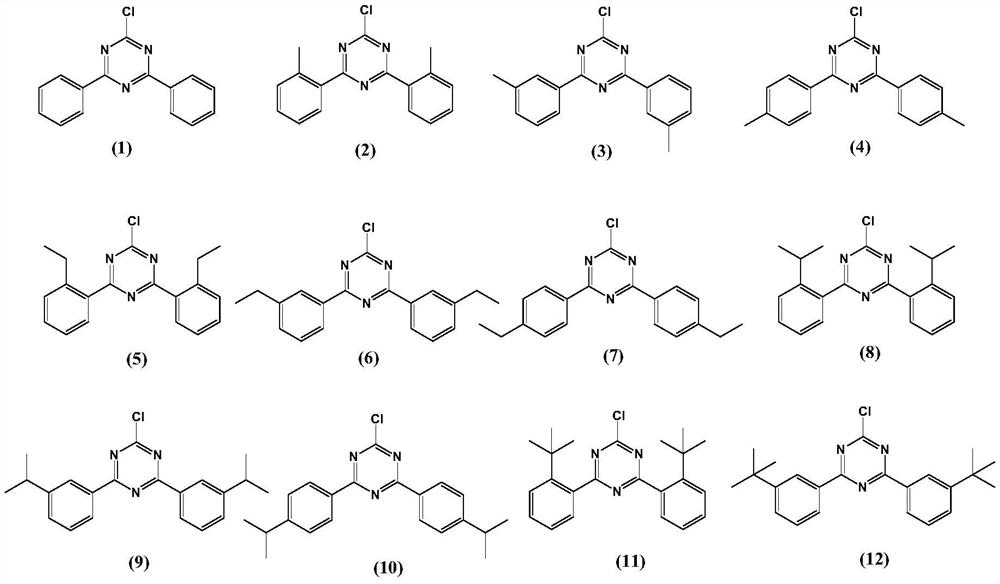
[0068] Under nitrogen, 400ml of tetrahydrofuran and 2-methylbromobenzene (yellow transparent liquid, this is the main raw material) (40g; 0.23mol) were successively added into a 1000ml three-necked flask. Start stirring, cool down to -70°C, react for 0.5 hours, then add sec-butyllithium (120ml, 0.3mol) dropwise, keep the temperature at -70°C for one hour, then quickly add cyanuric chloride (white acidic crystals, made here Main raw material) (22g, 0.12mol), heat preservation reaction for two hours.
[0069] Post-processing: the reaction solution is heated to -40°C and quenched by adding 400ml of 1mol / L dilute hydrochloric acid, extracting the system with 400ml of ethyl acetate, separating the organic phase, drying the organic phase and concentrating under reduced pressure (the organic phase is dried with anhydrous magnesium sulfate Dehydration, after removing magnesium sulfate with Buchner funnel suction filtration, liquid carries out concentration and removes solvent with rot...
Embodiment 2
[0071] Under nitrogen, 400ml of tetrahydrofuran and 3-methylbromobenzene (40g; 0.23mol) were successively added into a 1000ml three-neck flask. Start stirring, cool down to -70°C, react for 0.5 hours, then add n-butyllithium (120ml, 0.3mol) dropwise, keep the temperature at -70°C for one hour, then quickly add cyanuric chloride (22g, 0.12mol), keep warm React for two hours.
[0072] Post-processing: the reaction solution is heated to -40°C and quenched by adding 400ml of 1mol / L dilute hydrochloric acid, extracting the system with 400ml of ethyl acetate, separating the organic phase, drying the organic phase and concentrating under reduced pressure (the organic phase is dried with anhydrous magnesium sulfate Dehydration, after removing magnesium sulfate with Buchner funnel suction filtration, liquid carries out concentration and removes solvent with rotary evaporator under negative pressure, obtains solid product after concentration), recrystallizes with sherwood oil (the thick...
Embodiment 3
[0074] Under nitrogen, 400ml of tetrahydrofuran and 4-tert-butylbromobenzene (49g; 0.23mol) were successively added into a 1000ml three-neck flask. Start stirring, cool down to -70°C, react for 0.5 hours, then add sec-butyllithium (120ml, 0.3mol) dropwise, keep the temperature at -70°C for one hour, then quickly add cyanuric chloride (22g, 0.12mol), keep warm React for two hours.
[0075] Post-processing: the reaction solution is heated to -40°C and quenched by adding 400ml of 1mol / L dilute hydrochloric acid, extracting the system with 400ml of ethyl acetate, separating the organic phase, drying the organic phase and concentrating under reduced pressure (the organic phase is dried with anhydrous magnesium sulfate Dehydration, after removing magnesium sulfate with Buchner funnel suction filtration, liquid carries out concentration and removes solvent with rotary evaporator under negative pressure, obtains solid product after concentration), recrystallizes with sherwood oil (the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com