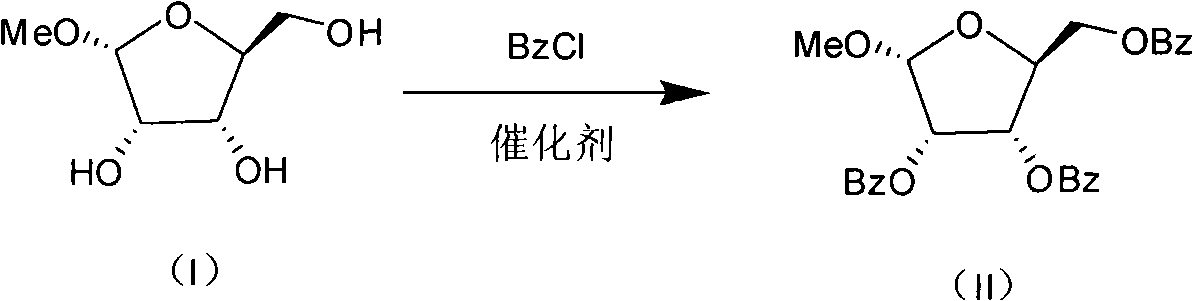
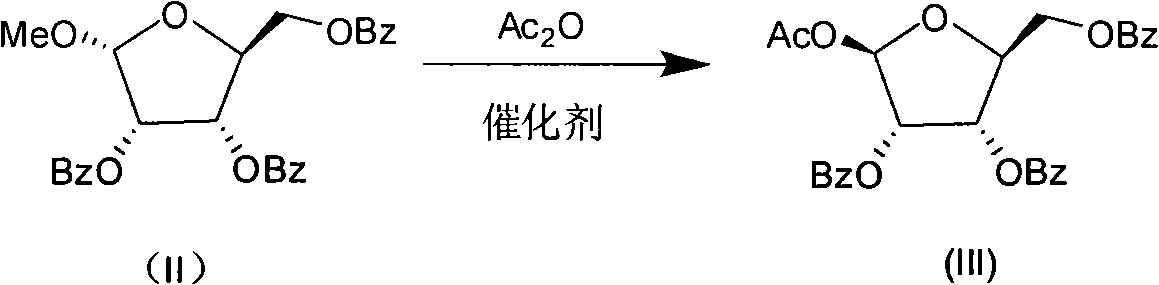
Production process for preparing 1-oxy-acetyl-2,3,5-3-benzoyl-beta-Lribofuranose
A ribofuranose and benzoyl technology, applied in the direction of sugar derivatives, sugar derivatives, esterified saccharides, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0014] The present invention will be further described below by way of examples.
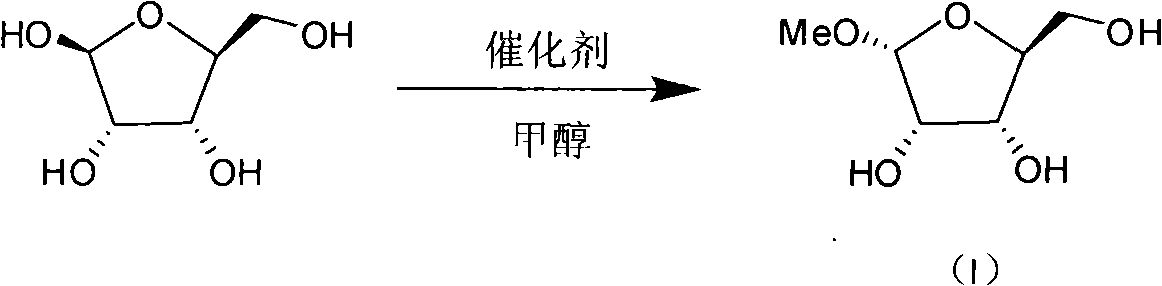
[0015] Step 1: Using L-ribose, methanol, hydrogen chloride, etc. as raw materials, synthesize 1-oxo-methyl-L-ribofuranose (I) through methylation. The mass ratio of L-ribose to methanol is 1:10-16, such as 1:12 or 1:14, preferably 1:12-13, and stirred at room temperature. After all the raw materials are dissolved, cool to -10°C and slowly introduce hydrogen chloride gas in an amount of 25% to 50% of the mass of L-ribose, such as 27% or 35% or 40%, most preferably 30% to 35%. After passing through, the reaction temperature is controlled at -15°C to 5°C, preferably -10°C to 1°C, and stirred for 10 to 18 hours, preferably 13 to 15 hours. TLC and HPLC tracked until the reaction of raw materials was complete. Slowly add pyridine with the same mass as ribose, stir and neutralize for 30 minutes. The crude product of 1-oxo-methyl-L-ribofuranose (I) can be obtained in a quantitative yield by high-vacu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com