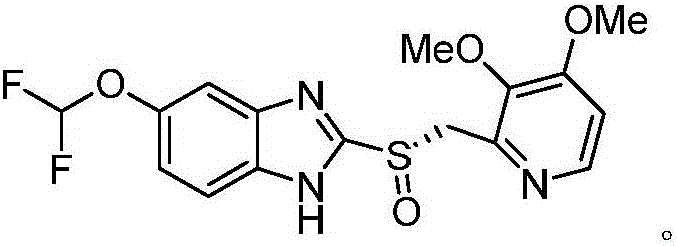
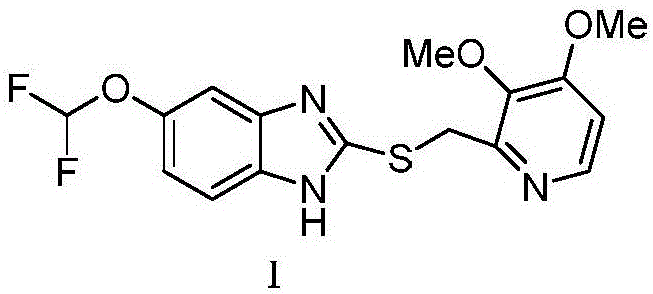
Synthesis process of (L)-pantoprazole
A synthesis process, pantoprazole technology, applied in the field of synthesis process of levo-pantoprazole, can solve the problems of poor selectivity, low yield, difficult post-treatment and purification, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Synthesis of Pantoprazole Thioether
[0031] In a 500ml flask, add 250ml of mixed solvent (mixed solvent is composed of tetrahydrofuran and water with a volume ratio of 3:1), and then 21.62g (100mmol) of 5-difluoromethoxy-2-mercapto-1H-benzimidazole , 2.54g (10mmol) of iodine, 97.75g of base (cesium carbonate, 300mmol) and 26.89g (120mmol) of 2-chloromethyl-3,4-dioxypyridine hydrochloride were added to the flask, stirred at 30-50°C Reacted for 1.5 hours, cooled to room temperature, extracted with dichloromethane, washed with water, recrystallized from petroleum ether / dichloromethane, and vacuum-dried to obtain 35.87g of pantoprazole sulfide shown in formula I, with a yield of 97.65% and a purity of 99.45%. .
Embodiment 2
[0033] Synthesis of Pantoprazole Thioether
[0034] In a 500ml flask, add 250ml of mixed solvent (mixed solvent is composed of tetrahydrofuran and water with a volume ratio of 3:1), and then 21.62g (100mmol) of 5-difluoromethoxy-2-mercapto-1H-benzimidazole , 1.27g (5mmol) of iodine, 31.8g of alkali (sodium carbonate, 300mmol) and 24.65g (110mmol) of 2-chloromethyl-3,4-dioxypyridine hydrochloride were added to the flask, stirred at 30-50°C Reacted for 2 hours, cooled to room temperature, extracted with dichloromethane, washed with water, recrystallized from petroleum ether / dichloromethane, and vacuum-dried to obtain 36.05 g of pantoprazole sulfide shown in formula I, with a yield of 98.14% and a purity of 99.27%. .
Embodiment 3
[0036] Synthesis of Pantoprazole Thioether
[0037] In a 500ml flask, add 250ml of mixed solvent (the mixed solvent is composed of tetrahydrofuran and water with a volume ratio of 2:1), and then 21.62g (100mmol) of 5-difluoromethoxy-2-mercapto-1H-benzimidazole , 1.78g (7mmol) of iodine, 65.16g of base (cesium carbonate, 200mmol) and 33.61g (150mmol) of 2-chloromethyl-3,4-dioxypyridine hydrochloride were added to the flask, stirred at 30-50°C Reacted for 2 hours, cooled to room temperature, extracted with dichloromethane, washed with water, recrystallized from petroleum ether / dichloromethane, and vacuum-dried to obtain 35.54 g of pantoprazole sulfide shown in formula I, with a yield of 96.73% and a purity of 99.49%. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com