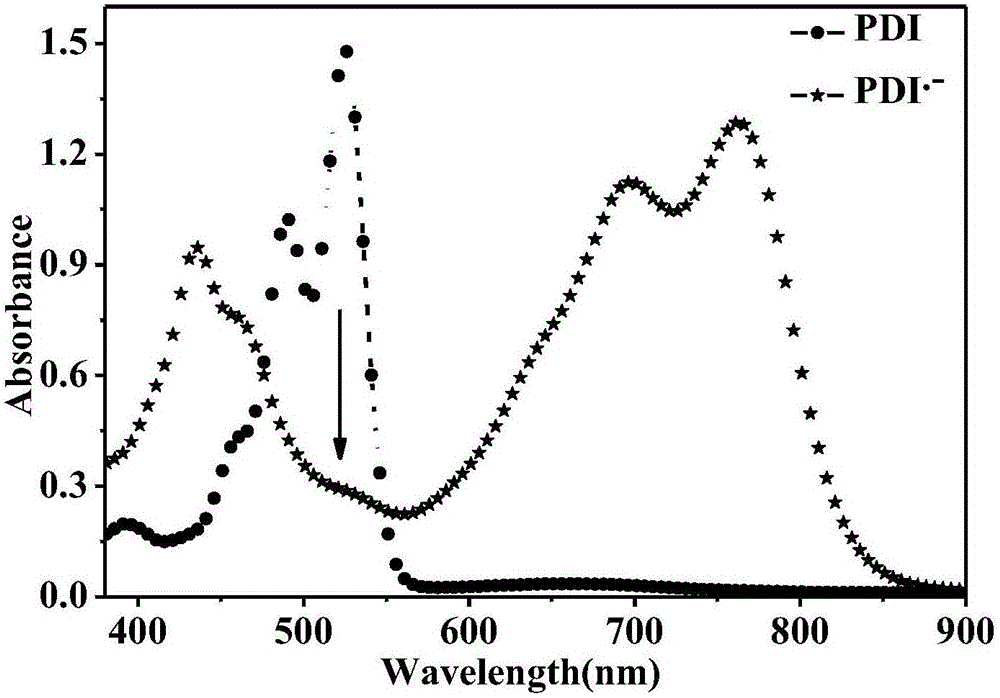
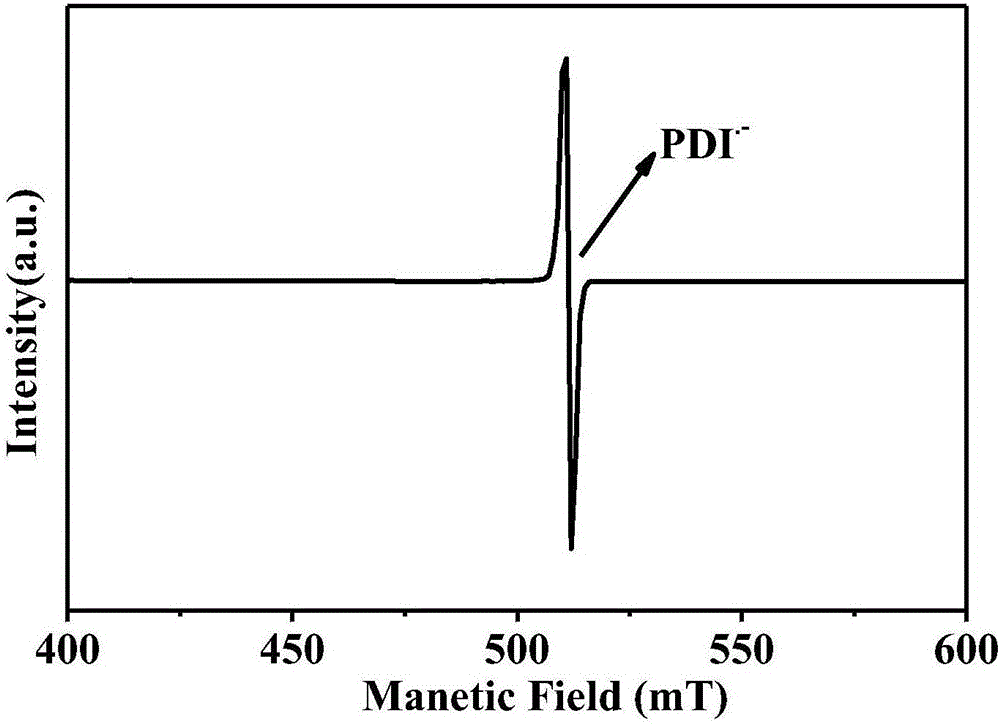
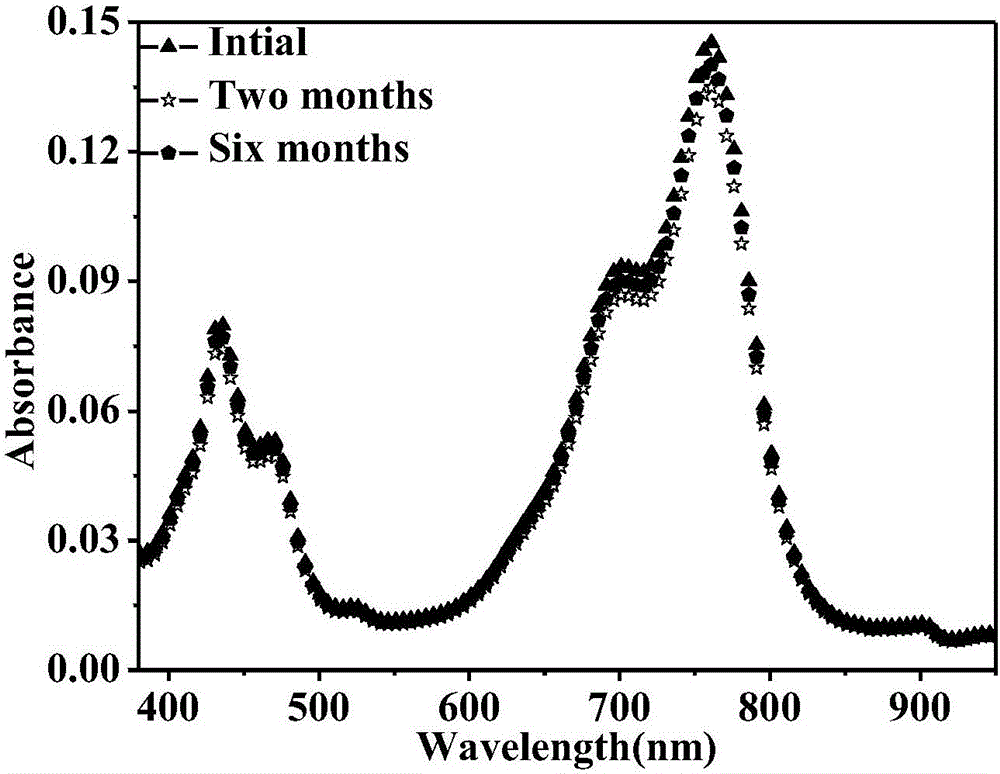
Reduced ionic salt of perylene bisimide and derivative of perylene bisimide and preparation method
A technology of perylene imide and derivatives is applied in the field of organic compounds and their electrochemical preparation, which can solve problems such as harsh requirements for raw materials and synthesis conditions, and achieve the effects of good air stability, strong stability and easy availability of raw materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Add 2.14 g of 3,4,9,10-perylenetetracarboxylic dianhydride (PTDA) (M=392) into 18 mL of concentrated (98%) H 2 SO 4(M=98, 1.836g / mL) in a three-necked flask, oscillated by 35Hz ultrasonic waves for 10min, then placed it on a heat-collecting constant temperature magnetic stirrer and added a condensing device, stirred at room temperature for 6h, and then adjusted the temperature to 80 ℃, reflux reaction, adding 0.053g I 2 (M=127) was used as a catalyst and about 0.577mL (3.12g / mL) of liquid bromine (M=160) was slowly added dropwise into it using a constant pressure dropping funnel. After reacting for 24 hours, dilute the reaction solution with concentrated sulfuric acid to 60% with distilled water, then suction filter to obtain a filter cake, and dry the filter cake in a vacuum oven at 90°C for 24 hours. The filter cake mainly contains the aforementioned three compounds a, b, and c, where a:b:c=7:2:1.
[0045] In a 100ml three-necked reaction flask, add respectively 0....
Embodiment 2
[0050] Add 2.09 g of 3,4,9,10-perylenetetracarboxylic dianhydride (PTDA) (M=392) into 18 mL of concentrated (98%) H 2 SO 4 (M=98, 1.836g / mL) in a three-necked flask, oscillated by 35Hz ultrasonic waves for 10min, then placed it on a heat-collecting constant temperature magnetic stirrer and added a condensing device, stirred at room temperature for 6h, and then adjusted the temperature to 80 ℃, reflux reaction, adding 0.052g I 2 (M=127) was used as a catalyst, and about 0.58 mL (3.12 g / mL) of liquid bromine (M=160) was slowly added dropwise into it using a constant pressure dropping funnel. After reacting for 24 hours, dilute the reaction solution with concentrated sulfuric acid to 60% with distilled water, then suction filter to obtain a filter cake, and dry the filter cake in a vacuum oven at 90°C for 24 hours. The filter cake mainly contains the aforementioned three compounds a, b, and c, where a:b:c=7:2:1.
[0051] In a 100ml three-necked reaction flask, add respectively...
Embodiment 3
[0056] Add 1.98 g of 3,4,9,10-perylenetetracarboxylic dianhydride (PTDA) into 18 mL of concentrated (98%) H 2 SO 4 In a three-necked flask, it was vibrated by 35Hz ultrasonic waves for 10min, then placed on a heat-collecting constant temperature magnetic stirrer and equipped with a condensing device, after stirring at room temperature for 6h, the temperature was adjusted to 80°C, and reflux reaction was added during which 0.052g I 2 As a catalyst, about 0.58 mL of liquid bromine was slowly added dropwise into it with a constant pressure dropping funnel. After reacting for 24 hours, dilute the reaction solution with concentrated sulfuric acid to 60% with distilled water, then suction filter to obtain a filter cake, and dry the filter cake in a vacuum oven at 90°C for 24 hours. The filter cake mainly contains the aforementioned three compounds a, b, and c, where a:b:c=7:2:1.
[0057] In a 100ml three-necked reaction flask, add 0.95g of bromoperylenetetraanhydride prepared by t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com