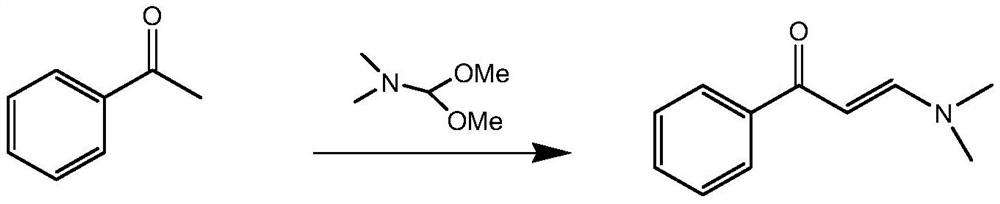
Preparation method and application of N-alpha-beta-containing unsaturated ketone compound
A ketone compound, unsaturated technology, applied in the field of drug synthesis, can solve the problems of difficult purification, high production cost, cumbersome process, etc., and achieve the effect of simple post-processing purification, reduced preparation cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
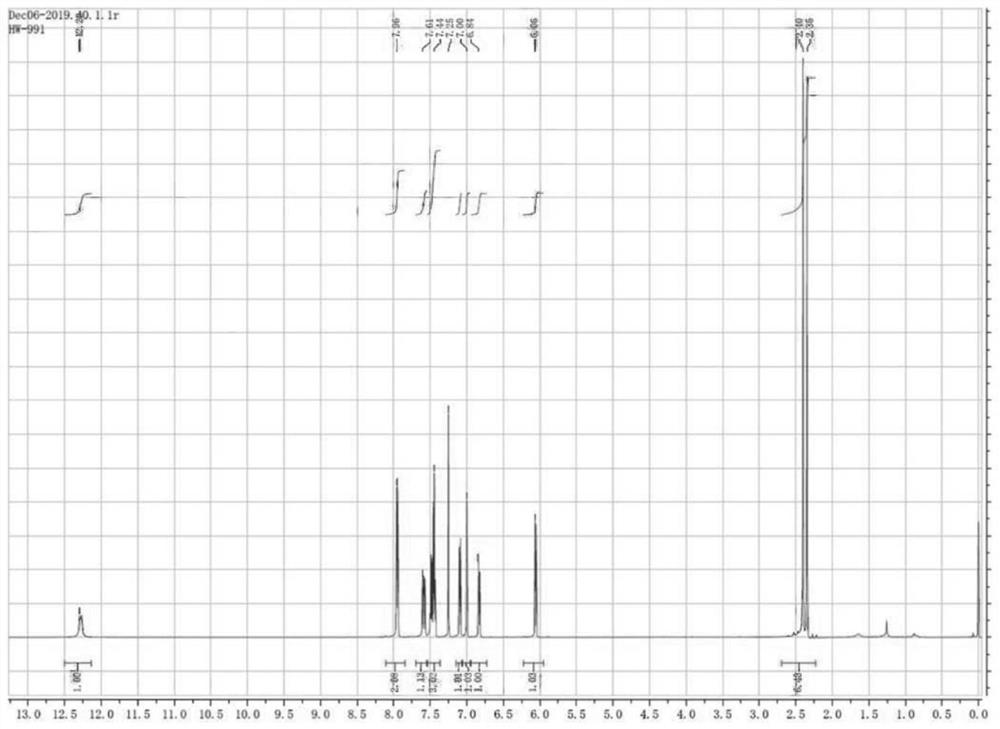
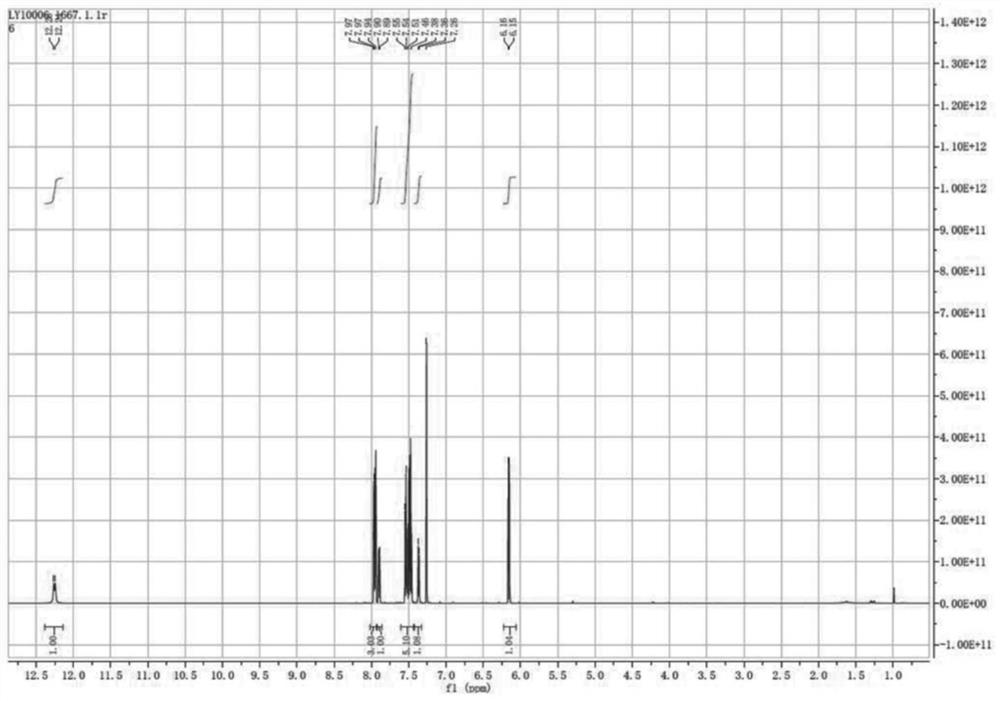
Image
Examples
Embodiment 1
[0025] (1)
[0026] The table of raw materials of table 1 embodiment 1 step (1)
[0027] materials Feeding amount M moles molar feed ratio Acetophenone 50g 120.15 0.416mol 1 DMF-DMA 77.4g 119.2 0.649mol 1.5
[0028] Mix acetophenone and DMF-DMA together according to the dosage in Table 1, heat up to the reflux point, and the reaction system turns yellow after 20 minutes, then cool down the reaction system to -10°C, precipitate solids, methyl tert-butyl ether Slurry to obtain a pure yellow solid. The product obtained after beating is 50-60g in total, and the yield has reached more than 80%.
[0029] (2)
[0030] The raw material table of table 2 embodiment 1 step (2)
[0031]
[0032] According to the raw material consumption in table 2, in Add water, then add nitroaniline, then add ferric chloride hydrate, stir overnight, solid precipitates, add water to quench, extract with EA, concentrate through the column, and beat with...
Embodiment 2
[0034] (1)
[0035] Table 3 The raw material list of embodiment 2 step (1)
[0036] materials Feeding amount M moles molar feed ratio Acetophenone 50g 120.15 0.416mol 1 DMF-DMA 77.4g 119.2 0.649mol 1.5
[0037] Mix acetophenone and DMF-DMA together according to the dosage in Table 3, heat up to the reflux point, the reaction system turns yellow after 20 minutes, cool down the reaction system to -10°C, precipitate solids, methyl tert-butyl ether Slurry to obtain a pure yellow solid. The product obtained after beating is 50-60g in total, and the yield has reached more than 80%.
[0038] (2)
[0039] The raw material table of table 4 embodiment 2 steps (2)
[0040]
[0041] According to the amount of raw materials in Table 4, the Add in water to dissolve, then add aniline, ferric chloride hydrate, the color becomes darker, stir overnight, a solid precipitates, add water to quench, extract with EA, concentrate through the colum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com