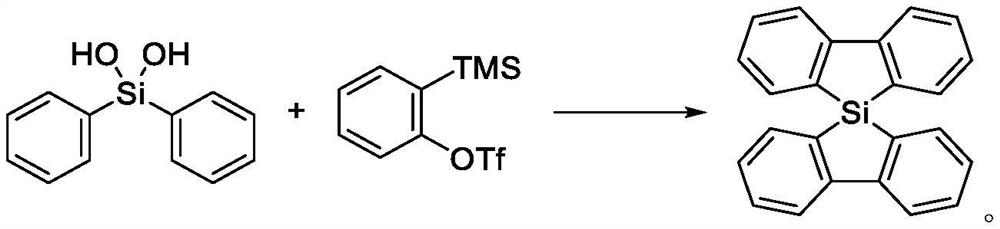
Method for synthesizing 5, 5-spirosilafluorene through C-H arylation cyclization reaction
A synthesis method and a technology of cyclization reaction, which are applied in the field of organic synthesis of spirobifluorene derivatives, can solve problems such as high cost of starting materials, harsh reaction conditions, and complicated synthesis steps, and avoid lithium reagents and halosilanes. The use, production cost is easy to control, and the effect of high reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: A kind of synthetic method of 5,5-spirosilafluorene
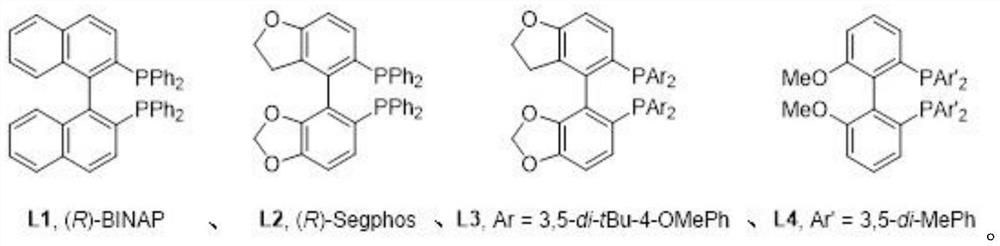
[0040] The synthesis steps include: under nitrogen protection, add 43.3g of diphenyldihydroxysilane (98%, 0.2mol), 131.3g of 2-(trimethylsilyl)phenyltrifluoromethanesulfonic acid into a 1000mL reaction flask Salt (97%, 0.44mol), 1.85g of Rh (PPh 3 ) 3 Cl (98%, 2.0mmol), 3.0g of ligand L3 (98%, 2.0mmol), 63.6g of sodium carbonate (99%, 0.6mol) and 500mL of o-dichlorobenzene; after feeding, heat up to 120°C , stirring speed 500rpm, heat preservation reaction for 18 hours, carry out C-H arylation and cyclization reaction, thin layer chromatography (TLC) monitors the progress of the reaction; after the reaction is completely completed, heat, cool to room temperature for filtration, washing, extraction, and solvent recovery of the organic layer , the crude product was crystallized from petroleum ether to obtain 53.1 g of 5,5-spirosilafluorene product, with a content of 99.1% and a yield of 83.2%; wherein t...
Embodiment 2
[0042] Embodiment 2: A kind of synthetic method of 5,5-spirosilafluorene
[0043] The synthesis steps include: under nitrogen protection, add 43.3g of diphenyldihydroxysilane (98%, 0.2mol), 131.3g of 2-(trimethylsilyl)phenyltrifluoromethanesulfonic acid into a 1000mL reaction flask Salt (97%, 0.44mol), 1.23g of [Cp * RhCl 2 ] 2 (98%, 2.0mmol), the ligand L1 (98%, 2.0mmol) of 1.25g, the sodium tert-butoxide (98%, 0.6mol) of 57.7g and the o-dichlorobenzene of 500mL; Feed intake finishes, be warming up to 140 ℃, stirring speed 500rpm, heat preservation reaction for 18 hours, carry out C-H arylation and cyclization reaction, and monitor the progress of the reaction by thin layer chromatography (TLC); after the reaction is completely completed, heat, cool to room temperature for filtration, washing, extraction, and the organic layer is precipitated and recovered Solvent, the crude product was crystallized from a petroleum ether / ethyl acetate mixed solution to obtain 46.8g of 5,5...
Embodiment 3
[0045] Embodiment 3: A kind of synthetic method of 5,5-spirosilafluorene
[0046] The synthesis steps include: under nitrogen protection, add 43.3g of diphenyldihydroxysilane (98%, 0.2mol), 179.0g of 2-(trimethylsilyl)phenyltrifluoromethanesulfonic acid into a 1000mL reaction flask salt (97%, 0.6mol), 0.93g of Rh (PPh 3 ) 3 Cl (98%, 1.0mmol), 1.3g of ligand L4 (98%, 1.0mmol), 162.9g of cesium carbonate (99%, 0.5mol) and 600mL of mesitylene; after feeding, the temperature was raised to 140°C, The stirring speed is 500rpm, the heat preservation reaction is carried out for 24 hours, and the C-H arylation and cyclization reaction is carried out, and the reaction progress is monitored by thin layer chromatography (TLC); after the reaction is completely completed, the heating is cooled to room temperature, filtered, washed, extracted, and the organic layer is desolvated to recover the solvent. The crude product was crystallized from a petroleum ether / ethyl acetate mixed solution t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com