A kind of preparation method of (s)-glycidyl phthalimide
A technology of glycerol phthalimide and toluene, applied in the field of medicine and biochemical industry, can solve the problems of low solubility of potassium phthalimide, unfavorable environmental protection and industrialized production, poor stability of epoxy ring, and the like, Achieve easy post-processing, easy control and implementation, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1: 2-((S)-3-chloro-2-hydroxypropyl)isoindoline-1,3-dione (V 1 ) preparation
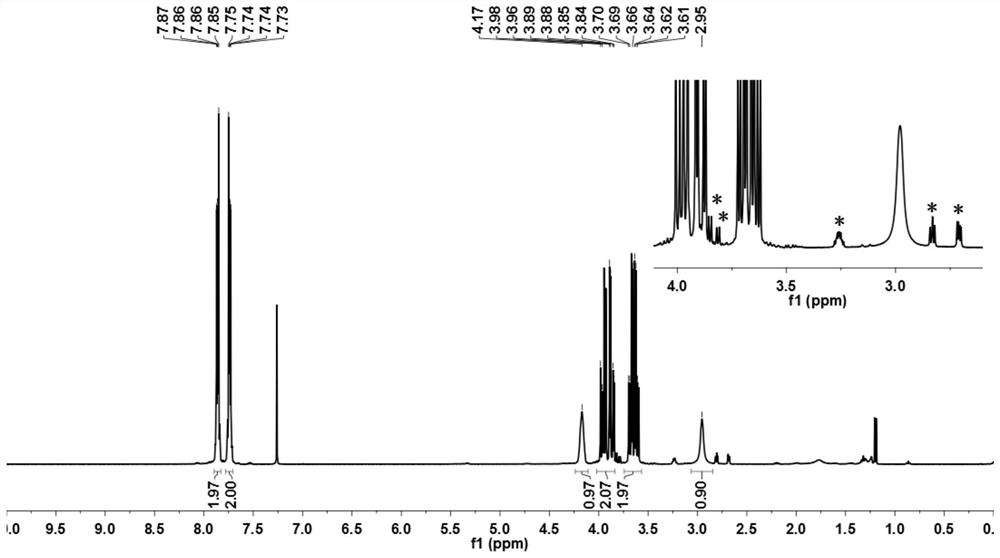
[0065] Into a 500 ml four-neck flask connected with stirring, thermometer and condenser, add 160 g of isopropanol, 29.6 g (0.20 mole) of phthalimide (Ⅲ), 27.8 g (0.30 mole) of (S) -1-Chloropropylene oxide (Ⅳ 1 ), 3.22 g (0.01 mole) of tetrabutylammonium bromide, heated, and kept stirring for 12 hours at an internal temperature of 43°C. Isopropanol was recovered by distillation under reduced pressure, 80 g of n-hexane was added to the residue, stirred, stirred at room temperature for 1 hour, filtered, the filter cake was washed with 20 g of n-hexane, and the filtrates were combined. Recover n-hexane and excess (S)-1-chloropropylene oxide by distillation under reduced pressure. After the filter cake was dried, 49.7 grams of crude product 2-((S)-3-chloro-2-hydroxypropyl)isoindoline-1,3-dione (V 1 ); directly used in Example 2 without further purification.
[0066] figure 1 Be the nu...
Embodiment 2
[0071] Embodiment 2: the preparation of (S)-glycidyl phthalimide (II)
[0072] In the 500 milliliter four-necked bottle that is connected with stirring, thermometer, add 200 grams of toluene, 49.7 grams of 2-((S)-3-chloro-2-hydroxypropyl) isoindoline- 1,3-diketone (Ⅴ 1 ), the stirring system is mixed evenly between 20-25°C. 36 grams of 30% sodium methoxide methanol solution was added dropwise thereto, and the addition was completed in 15 minutes. The reaction was stirred at 25°C for 0.5 hours. Filter, add 10 gram ice waters in the filtrate, distill and reclaim toluene, recrystallize residue with 100 gram ethanols, obtain 34.6 gram (S)-glycidyl phthalimides, yield 85.1% (in the form of phthalimide Formimide is calculated as starting material). Optical purity 99.2% ee.
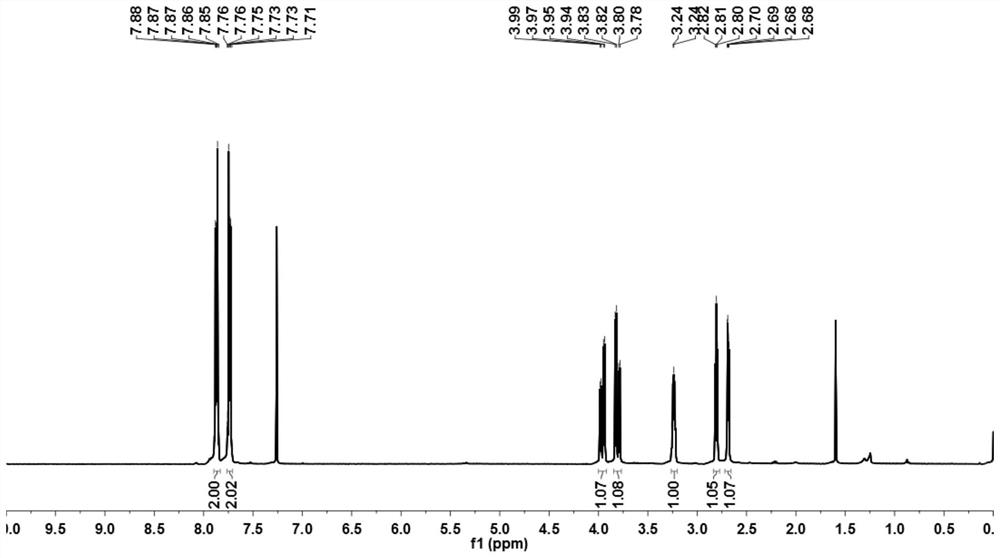
[0073] image 3 The proton nuclear magnetic spectrum (deuterated CDCl 3 );
[0074] The NMR data of the product are as follows:
[0075] 1 HNMR (400MHz, deuterated CDCl 3 )δ: 2.68(dd, J=4.8, 2.5Hz, 1H...
Embodiment 3
[0079] Example 3: 2-((S)-3-bromo-2-hydroxypropyl)isoindoline-1,3-dione (V 2 ) preparation
[0080] Add 160 g of isopropanol, 29.6 g (0.20 mol) of phthalimide (Ⅲ), 40.8 g (0.30 mol) of (S) -1-Bromopropylene oxide (Ⅳ 2 ), 3.22 grams (0.01 mol) of tetrabutylammonium bromide, heated, and kept stirring for 10 hours at an internal temperature of 43°C. Isopropanol was recovered by distillation under reduced pressure, 80 g of n-hexane was added to the residue, stirred, stirred at room temperature for 1 hour, filtered, the filter cake was washed with 20 g of n-hexane, and the filtrates were combined. Recover n-hexane and excess (S)-1-bromopropylene oxide by distillation under reduced pressure. After the filter cake was dried, 59.8 grams of crude product 2-((S)-3-bromo-2-hydroxypropyl)isoindoline-1,3-dione (V 2 ), was directly used in Example 4 without further purification. 2-((S)-3-bromo-2-hydroxypropyl)isoindoline-1,3-dione (V 2 ) and the (S)-glycidyl phthalimide (II) content of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com