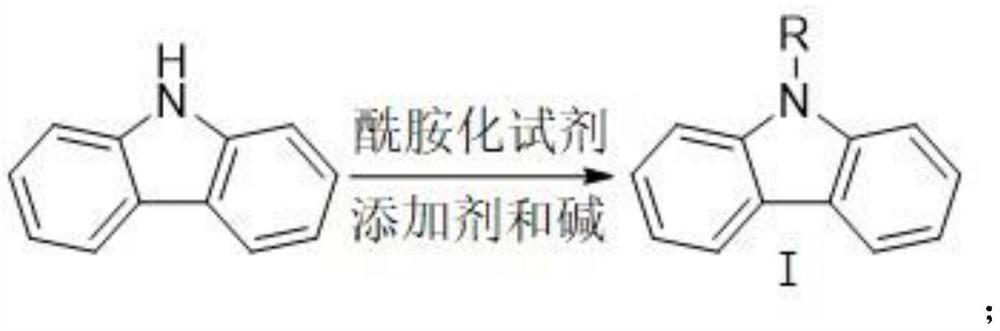
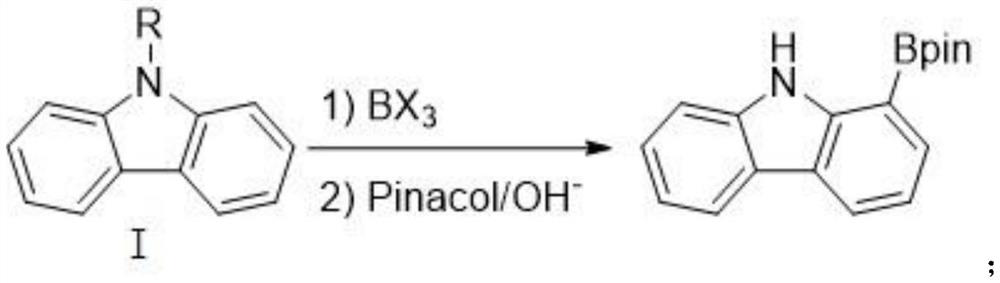
Method for synthesizing 1-carbazole-boronic acid pinacol ester through ortho-oriented boronation of amide
A technology for the synthesis of pinacol esters and compounds, which is applied in the field of carbazole derivatives synthesis, can solve the problems of complicated operation, low product yield, difficult purification and the like, and achieves avoiding reaction conditions, high product yield and mild reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: A kind of synthetic method of 1-carbazole-boronic acid pinacol ester
[0042] Step comprises: (1) under the condition of 0 ℃, add the carbazole (99%, 0.2mol) of 33.4g, the catalyst DMAP (98%, 0.04mol) of 4.9g, the triethylamine of 40.5g ( 98%, 0.4mol), 400mL of dichloromethane, after the system was stabilized at 0°C, 100mL of pivaloyl chloride (98%, 29.5g, 0.24mol) in dichloromethane was added dropwise; React at room temperature for 6 hours. After the reaction, add 100 mL of water to the organic layer and wash three times. Dichloromethane is recovered by distillation under reduced pressure. Concentrate and crystallize to obtain 48.5 g of N-pivaloyl carbazole as a white solid N-acyl carbazole intermediate, with a yield of 96.5%. (2) under the protection of nitrogen, add the dichloromethane of the N-pivaloyl carbazole (99%, 0.193mol) of synthesis 48.5g, 300mL in the reaction flask, drop boron tribromide (99%, 60.0g, 0.24mol) of dichloromethane solution 100m...
Embodiment 2
[0044] Embodiment 2: A kind of synthetic method of 1-carbazole-boronic acid pinacol ester
[0045] The steps include: (1) at -10°C, add 33.4g of carbazole (99%, 0.2mol), 4.9g of catalyst DMAP (98%, 0.04mol), 600mL of tetrahydrofuran to the reaction flask, and stir for 30 minutes Add 16.0g of NaH (60%, 0.4mol) again, after the temperature of the system is stable, rise to room temperature and react for 1 hour, then add dropwise 200mL of tetrahydrofuran solution of benzoyl chloride (98%, 43.0g, 0.30mol); , warmed up to room temperature and reacted for 4 hours; after the reaction, the organic layer was washed once with saturated brine and once with water, and tetrahydrofuran was recovered by distillation under reduced pressure, concentrated and crystallized to obtain a white solid N-acyl carbazole intermediate N-benzoyl carbazole 50.3 g, yield 92.7%;
[0046] (2) Under the protection of nitrogen, add 50.3g of synthesized N-benzoylcarbazole (99%, 0.185mol), 300mL of dichloromethan...
Embodiment 3
[0047] Embodiment 3: A kind of synthetic method of 1-carbazole-boronic acid pinacol ester
[0048] Step comprises: (1) under the condition of 0 ℃, add the carbazole (99%, 0.2mol) of 33.4g, the catalyst DMAP (98%, 0.04mol) of 4.9g, the triethylamine of 60.8g ( 98%, 0.6mol), 400mL tetrahydrofuran, stirred for 30 minutes, added dropwise 100mL of di-tert-butyl dicarbonate (98%, 49.0g, 0.22mol) tetrahydrofuran solution; after feeding, stirred for 30 minutes, and reacted at room temperature for 6 hours; After the reaction, the organic layer was washed once with saturated brine and once with water, and tetrahydrofuran was recovered by distillation under reduced pressure, and 100 mL of n-hexane was added for recrystallization to obtain 51.8 g of N-tert-butoxycarbonyl carbazole as a white solid N-acyl carbazole intermediate. Rate 96.8%;
[0049] (2) Under nitrogen protection, add 51.8g of synthesized N-tert-butoxycarbonylcarbazole (99%, 0.194mol), 300mL of dichloromethane in the react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com