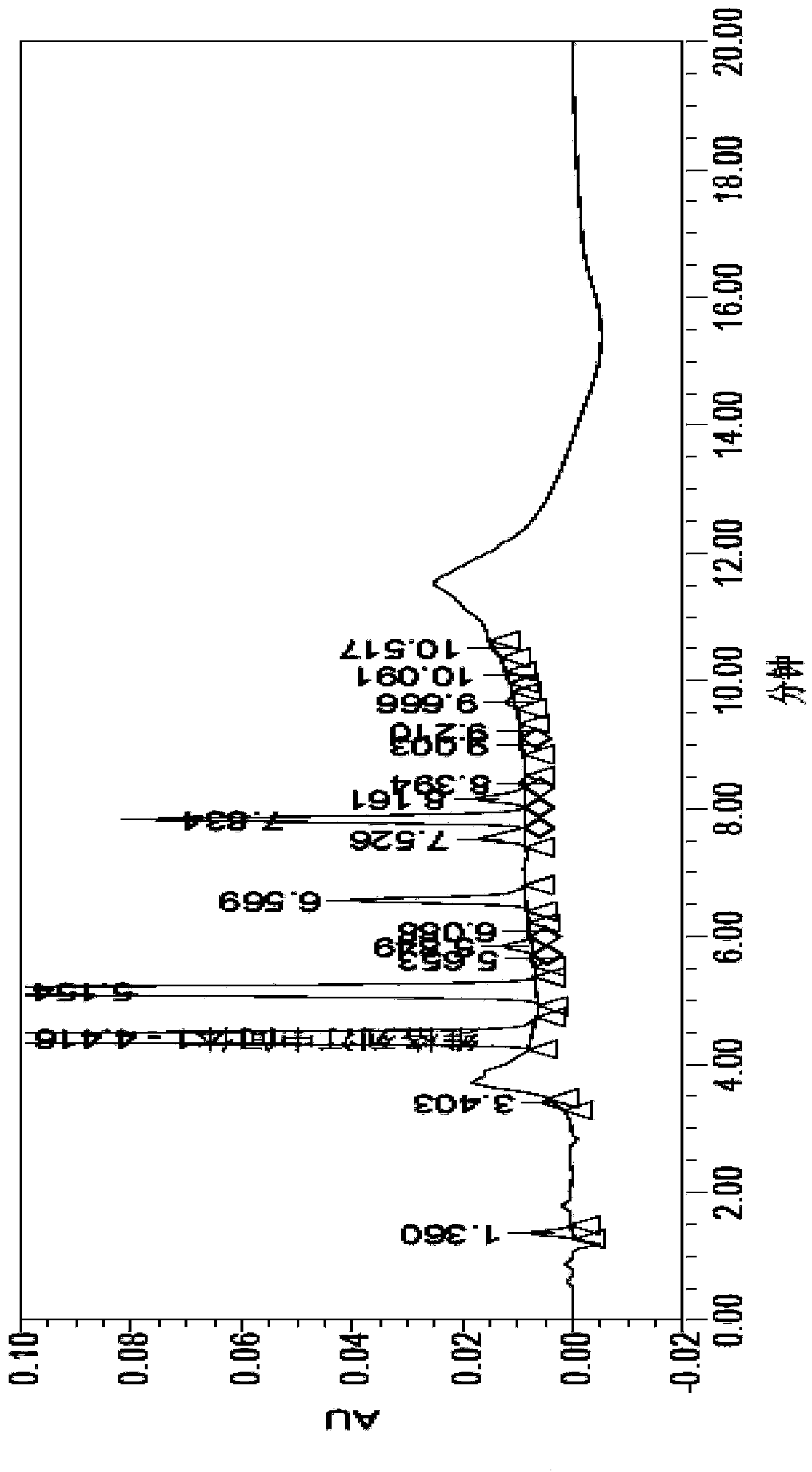
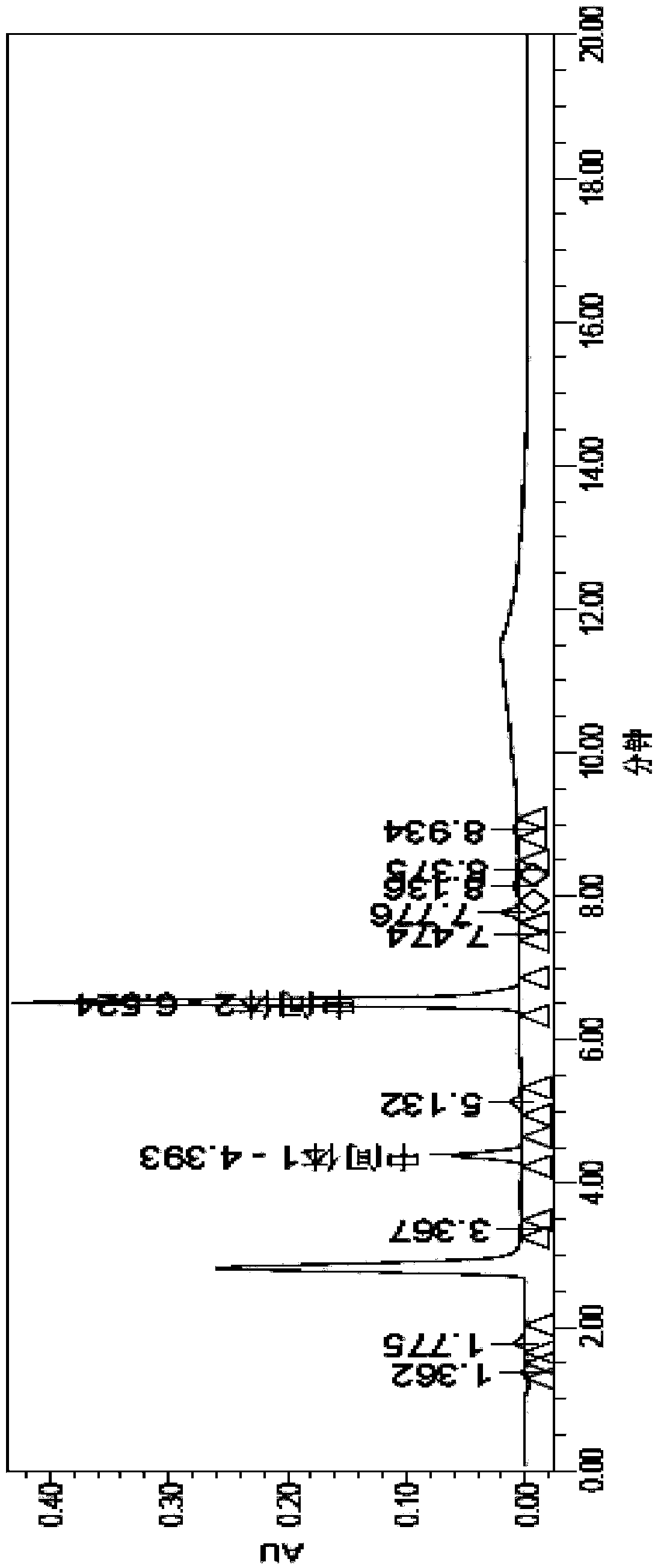
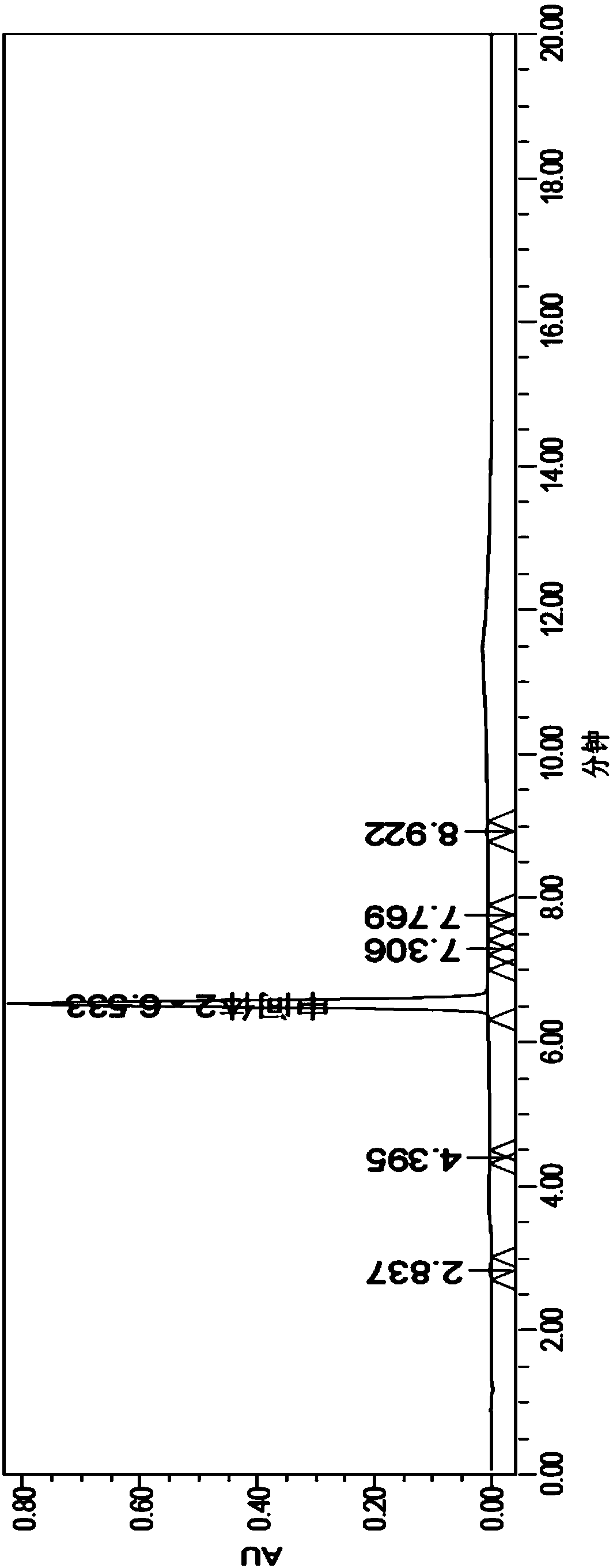
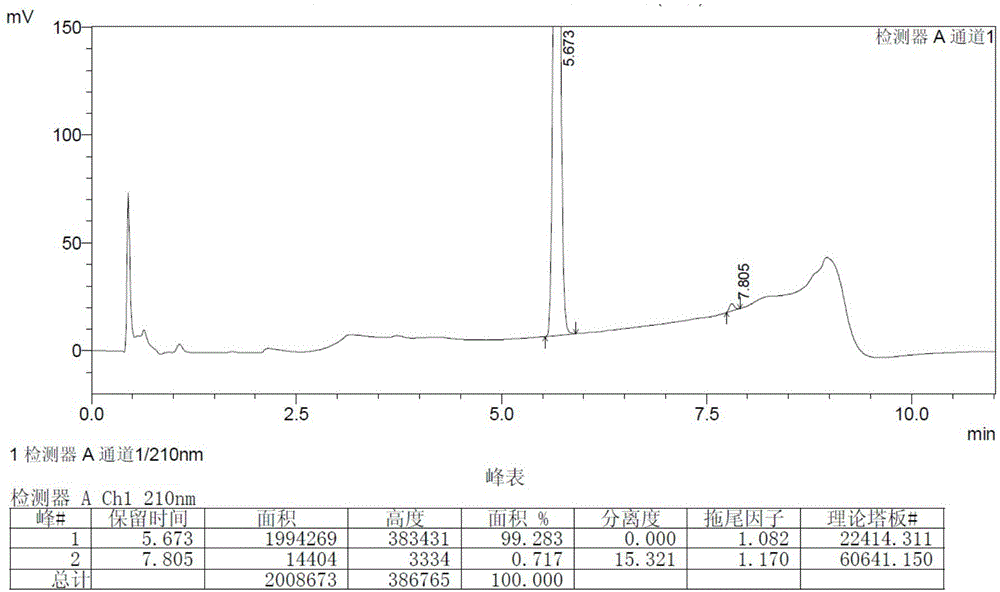
Patents
Literature
39 results about "1-adamantanol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
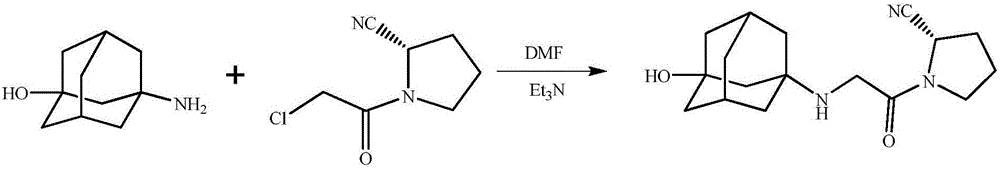
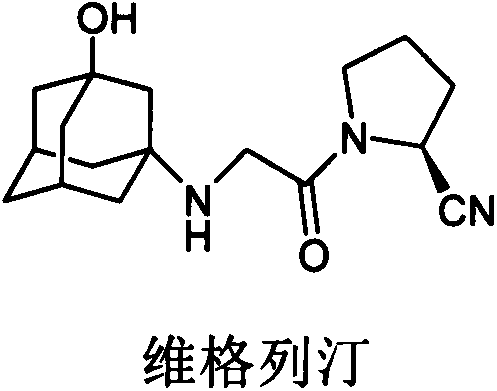
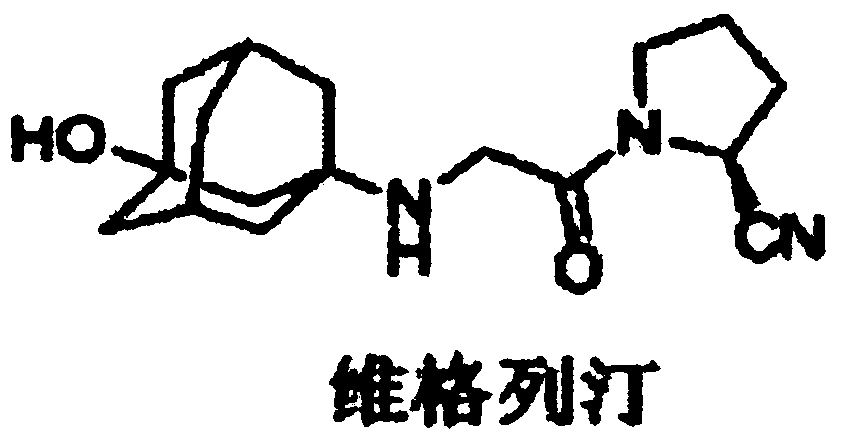
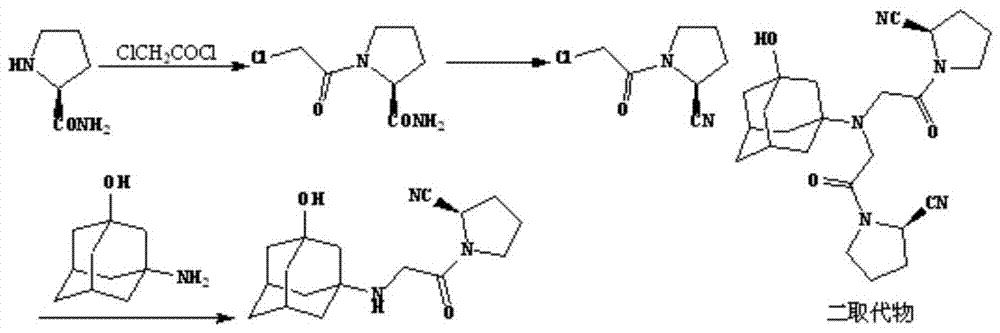
Synthetic process of vildagliptin
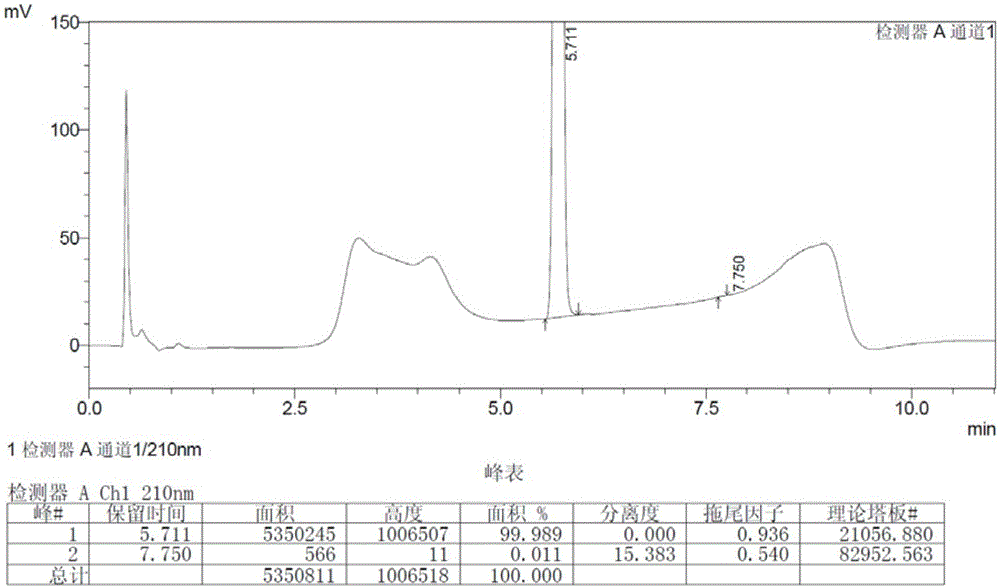
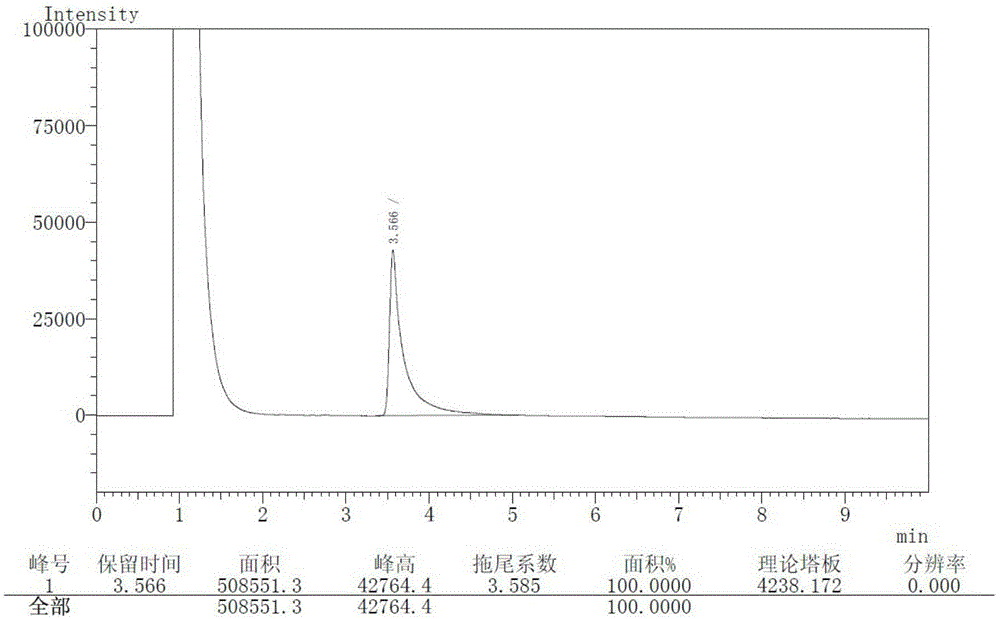
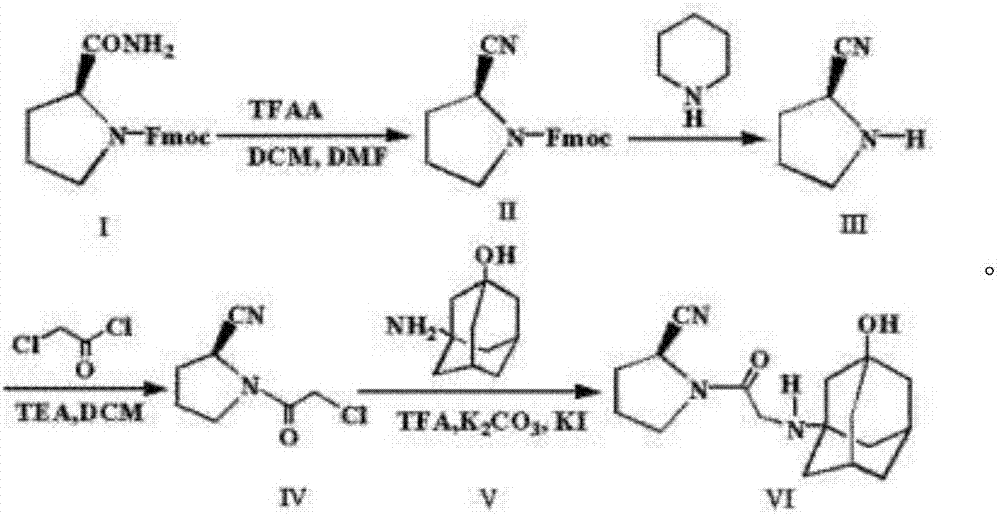
InactiveCN104326961AReduce pollutionSimple preparation processOrganic chemistryBulk chemical productionPyrrolidineVildagliptin
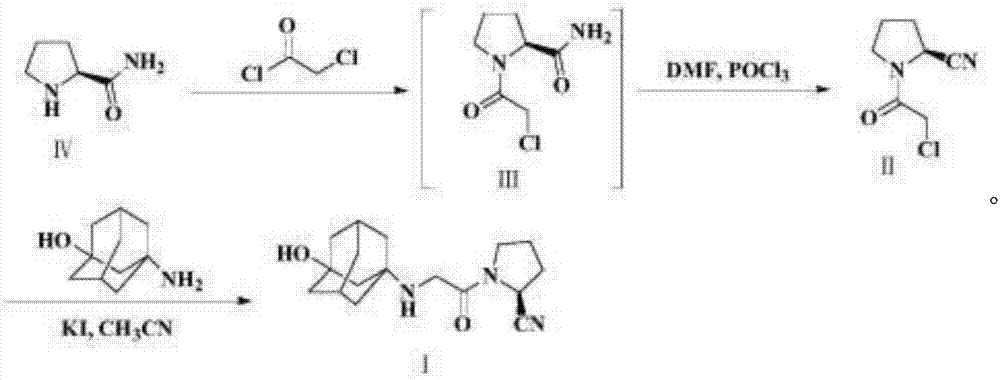
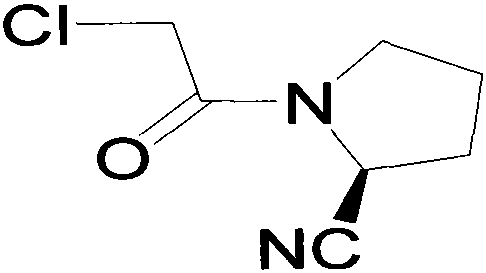
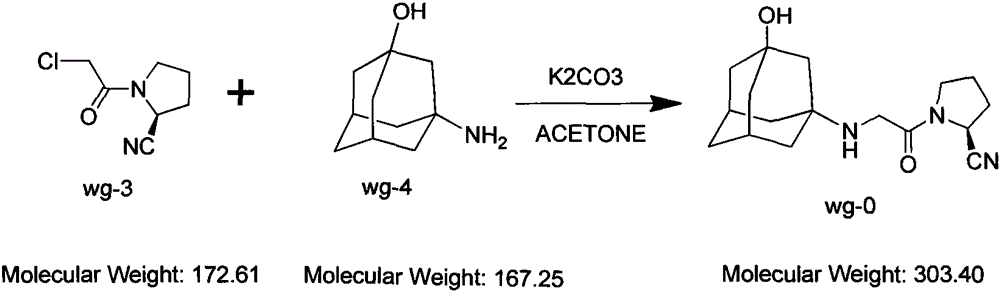
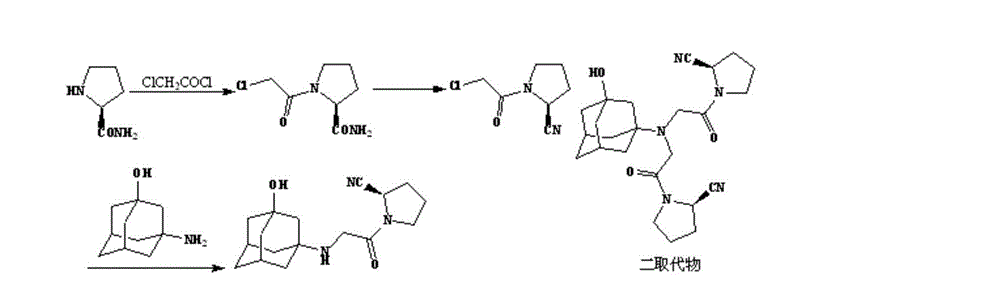
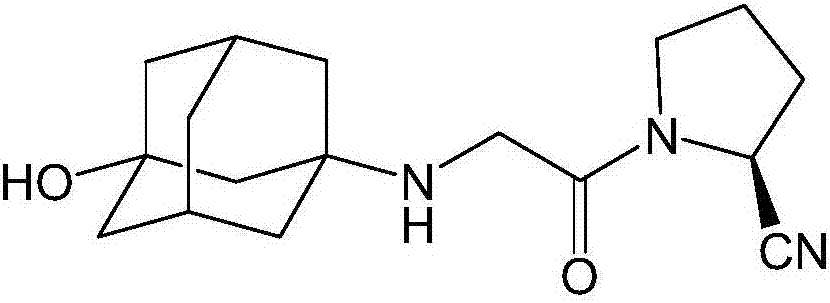
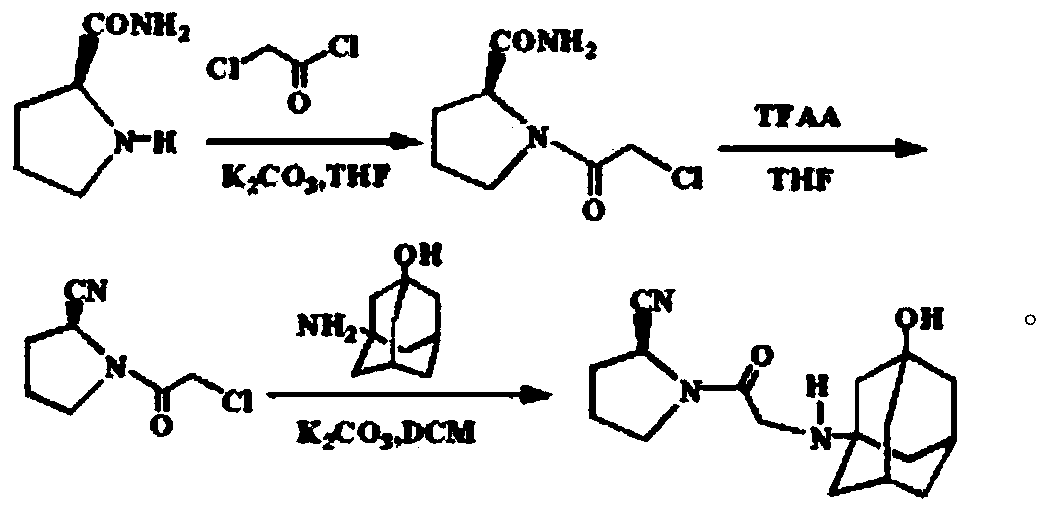
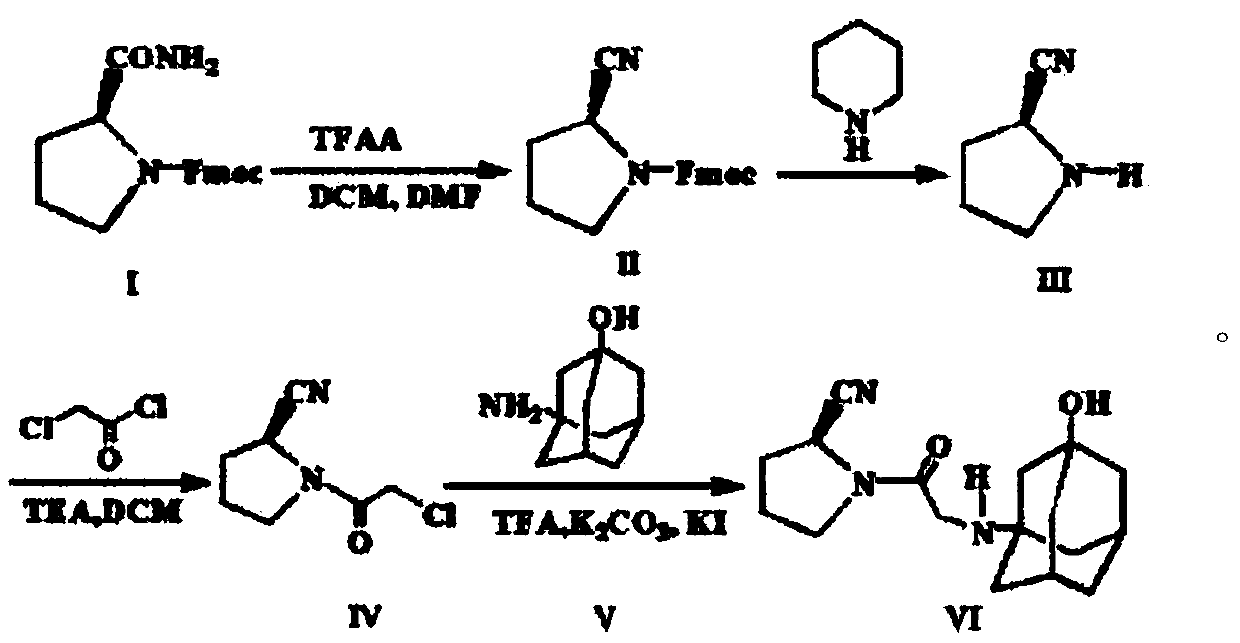
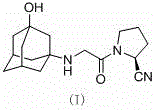
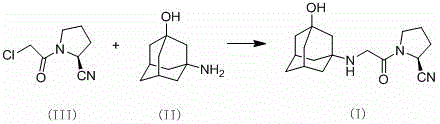
The invention belongs to the field of drug synthesis and discloses a novel method for preparing vildagliptin. The process comprises the following steps: dehydrating N-fluorenylmethoxy carbony-L-prolinamide (raw material) to form nitrile; removing the Fmoc- protective group and carrying out N-chloracetylation to obtain an intermediate (S)-1-(chloracetyl)-2-cyan pyrrolidine; further carrying out condensation on the intermediate and 3-amino-1-adamantanol to obtain a coarse vildagliptin product; and recrystallizing to obtain a refined vildagliptin product. The process flow used for synthesizing vildagliptin is simple in method and low in impurity content, thereby facilitating industrial production of vildagliptin.
Owner:HAINAN ZHONGHE PHARM CO LTD
Preparation method of high-purity vildagliptin
InactiveCN105085360AReduce contentImprove technical effectOrganic chemistrySynthesis methodsPotassium iodine
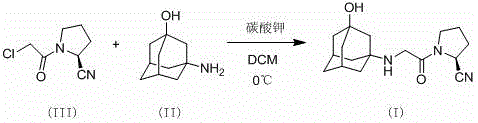
The invention discloses a preparation method of high-purity vildagliptin, which comprises the following steps: carrying out nucleophilic substitution reaction on an intermediate (2S)-1-(2-chloracetyl)-2-pyrrolidine formonitrile and 3-amino-1-adamantanol in a certain reaction solvent by using potassium iodide as a catalyst and potassium carbonate as an acid-binding agent, carrying out after-treatment to obtain grease, and carrying out solvent crystallization to obtain a vildagliptin crude product; and refining the vildagliptin crude product by using a purified solvent to obtain the refined product. The synthesis method by controlling the vildagliptin crude product obtains favorable technical effects, and can obviously lower the impurity content in the vildagliptin crude product. A refinement technique different from the documents is adopted, and isopropanol and 2-butanone impurity removal is performed to obtain favorable technical effects, thereby obviously lowering the content of the main impurity 3-amino-1-adamantanol and vildagliptin bis-substituted impurity in the vildagliptin crude product. The vildagliptin purity detected by HPLC (high performance liquid chromatography) and GC (gas chromatography) is up to 99.8% or above, and the contents of the two impurities are respectively lower than 0.1%.
Owner:NANJING UNIV OF SCI & TECH
Preparation method for Vildagliptin
The invention relates to a preparation method for Vildagliptin. The preparation method comprises the following steps: using L-prolinamide as a raw material, performing the dehydration reaction with cyanuric chloride, and generating (S)-2-cyanopyrrolidine; performing the salt forming reaction to the (S)-2-cyanopyrrolidine and hydrogen chloride, to obtain intermediate-1; enabling the intermediate-1 to react with chloroacetic acid under the conditions of using EDCI as a condensing agent, using HOBt as a catalyst, and using DIEA as an acid-binding agent, to obtain intermediate-2; enabling the intermediate-2 to react with 3-amino-1-adamantanol, to obtain the Vildagliptin, filtering, concentrating, crystallizing, and re-filtering to obtain a Vildagliptin crude product; and preparing the Vildagliptin finished product by the acetone refining. The preparation method is capable of providing a new method, and reducing the generation of by-products in each step. The content of the disubstitution product of the Vildagliptin by-product and the 3-amino-1-adamantanol are reduced to less than 0.1%, the yield and purity of the Vildagliptin are effectively improved, the raw materials are easily obtained, the conditions are moderate, and the preparation method is suitable for the industrial large-scale production.
Owner:HEBEI MEDICAL UNIVERSITY
Method for producing 1-adamantane ethanol
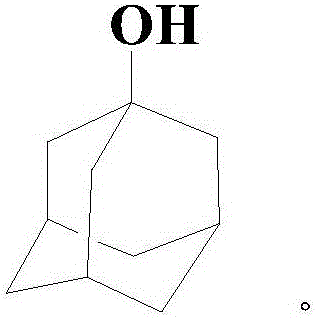
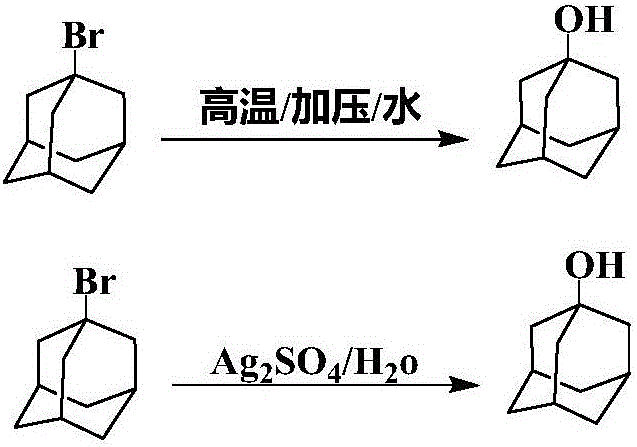
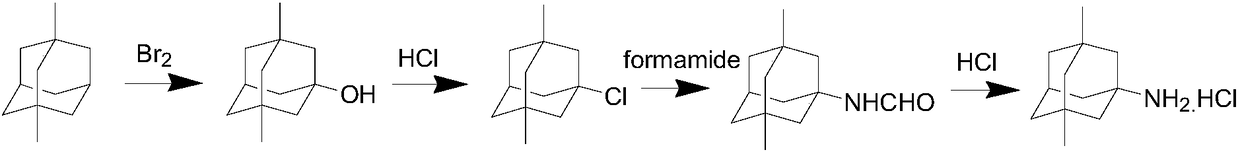
The invention relates to a method for producing a 1-adamantanol product which is prepared by subjecting adamantane to bromination reaction and hydrolysis reaction. Bromine is used as a halogenating agent, and the dosage is one to ten times of the adamantane in mole number. A is used as a bromination catalyst, and the dosage is 0.5 percent to 5 percent of the weight of the adamantane. The temperature of the bromination reaction is 0 DEG C to 100 DEG C, and the reaction pressure is normal pressure. The invention is characterized in that the hydrolysis temperature of 1-bromine adamantane is 100 DEG C to 150 DEG C, and the reaction pressure is 0-3atm. The purity of the 1-adamantanol obtained by the invention is more than or equal to 99.5 percent, and the yield is more than 95 percent.
Owner:YIBIN CHEMIST PHARMACHEM CO LTD
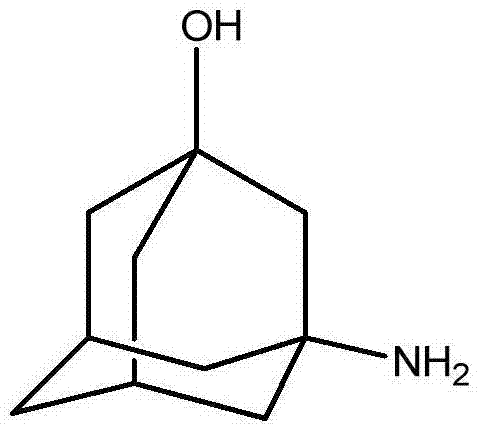
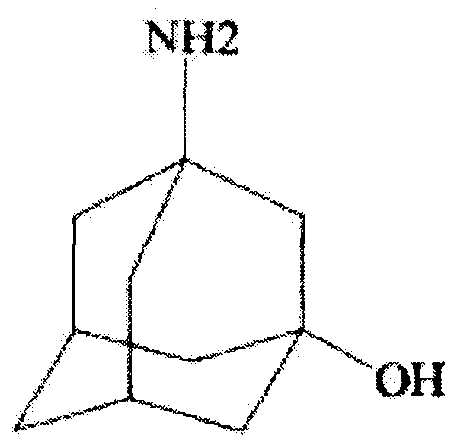
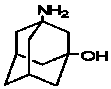
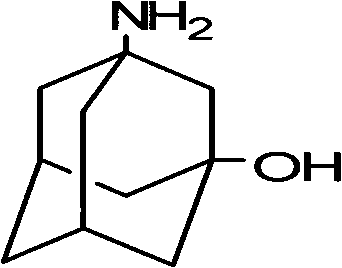
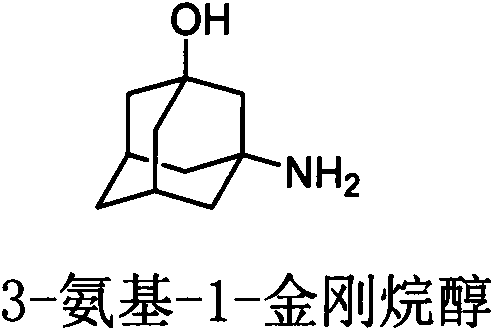
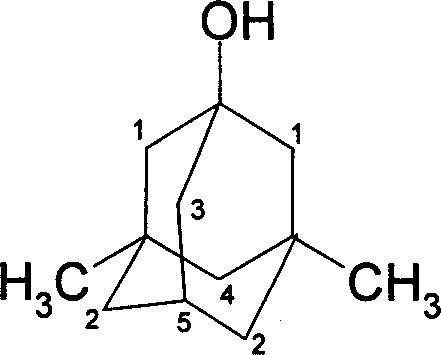
Preparation method of 3-amino-1-adamantanol
ActiveCN104761456ARaw materials are easy to getEasy to operateOrganic compound preparationAmino-hyroxy compound preparationNitration1-adamantanol
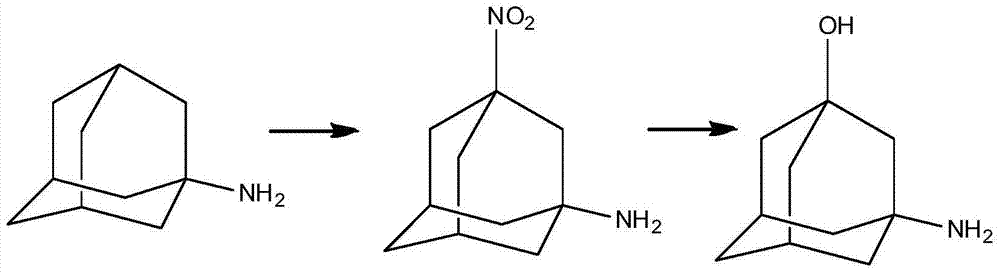
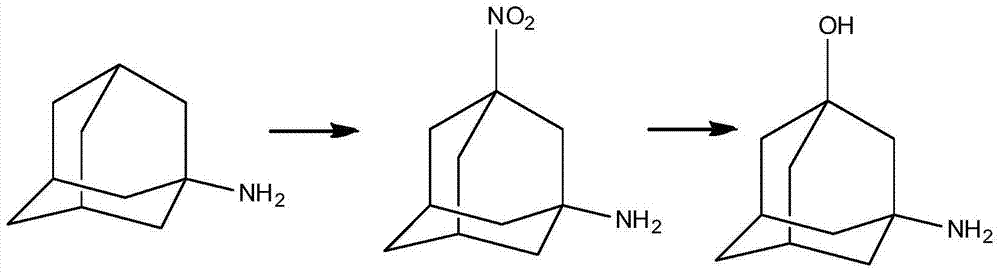
The invention discloses a preparation method of 3-amino-1-adamantanol, which comprises following steps: (1) adding amantadine or a salt thereof to sulfuric acid at 10-30 DEG C and adding dropwisely a mixed acid to perform a nitration reaction to obtain a reaction liquid; (2) adding the reaction liquid to water and mixing the reaction liquid with water to obtain a mixed solution; (3) performing a hydroxylation reaction under the effect of an alkaline to obtain the 3-amino-1-adamantanol . The preparation method employs the raw material being easy to obtain, is simple in operations, is environmental-protective, is low in cost, is high in yield which is generally higher than 80%, maximally 90.1%, and is more suitable for industrial production.
Owner:SHANGHAI VIWIT PHARMA CO LTD
Improved industrialization technology for preparing Vildagliptin
InactiveCN105153004ABroad developmentThe value of a wide range of applicationsOrganic chemistryFiltrationDouble salt
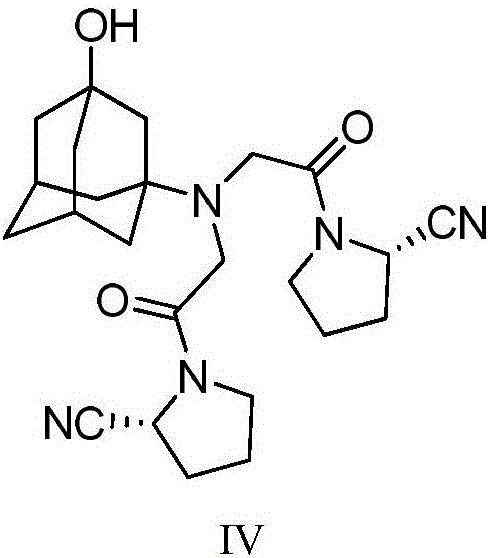
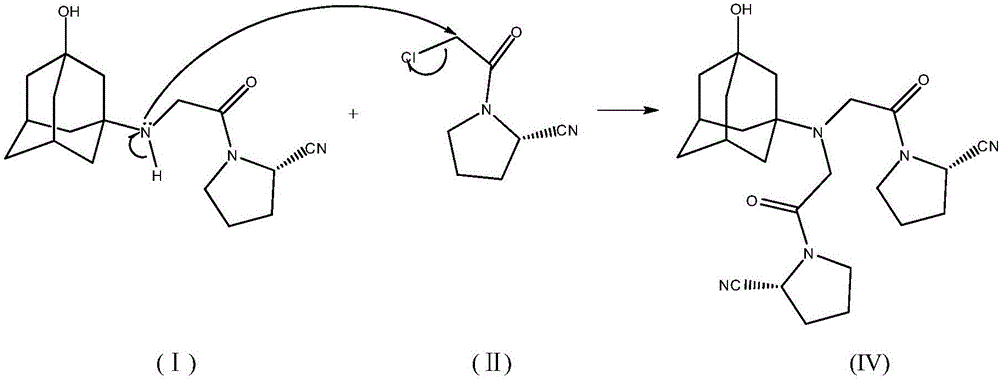
The invention discloses a novel preparation method of Vildagliptin. The preparation method comprises that L-prolinamide as a raw material, chloroacetyl chloride and tetrahydrofuran undergo an acylation reaction, the reaction product is subjected to suction filtration, the filtrate and trifluoroacetic anhydride directly undergo a dehydration reaction without filtrate separation purification to produce (-)-(2S)-1-chloroacetylpyrrolidine-2-carbonitrile (II), the refined (-)-(2S)-1-chloroacetylpyrrolidine-2-carbonitrile, 3-amino-1-adamantanol (III), potassium carbonate and potassium iodide undergo a reaction to produce (-)-(2S)-1-[[(3-hydroxytricyclo[3.3.1.1[3,7]]dec-1-yl)amino]acetyl]pyrrolidine-2-carbonitrile (I) in acetone, and the (-)-(2S)-1-[[(3-hydroxytricyclo[3.3.1.1[3,7]]dec-1-yl)amino]acetyl]pyrrolidine-2-carbonitrile (I) is refined by calcium double salt, ethyl acetate and butanone to form pure Vildagliptin (compound wg-0). The improved synthesis technology has the advantages of less reaction steps, operation simpleness, after-treatment simpleness, low employee cost, equipment cost and time cost, high yield, high product quality and industrialization feasibility.
Owner:BEIJING KCODE PHARMA R &D CO LTD
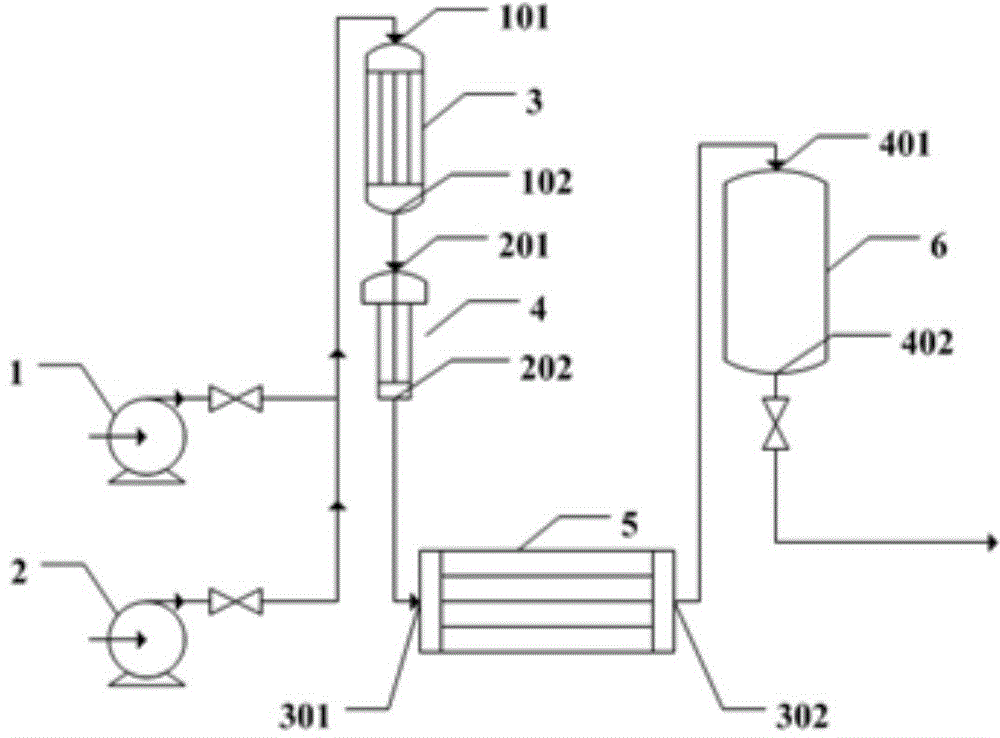
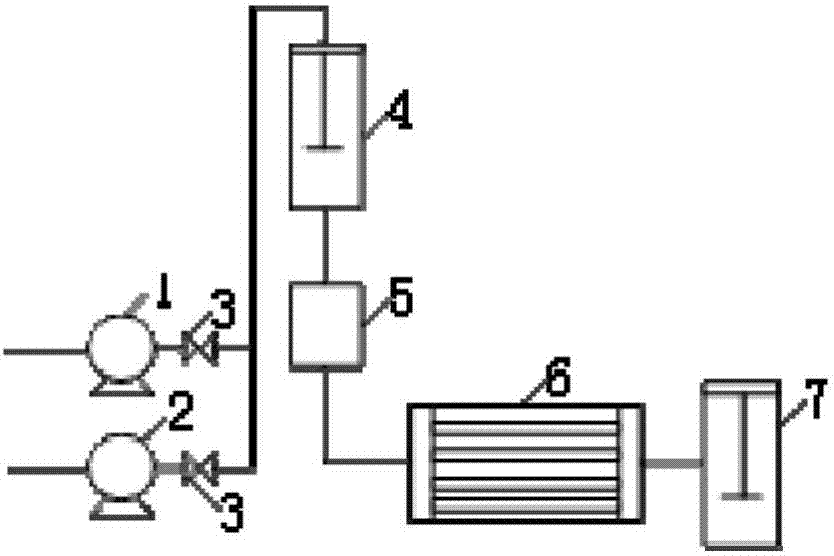
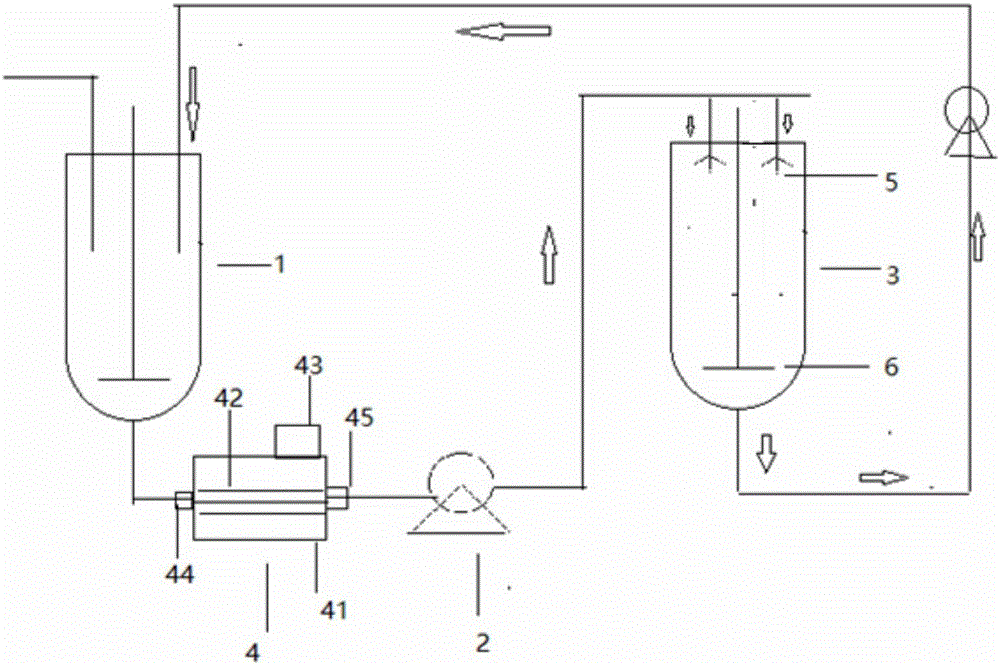
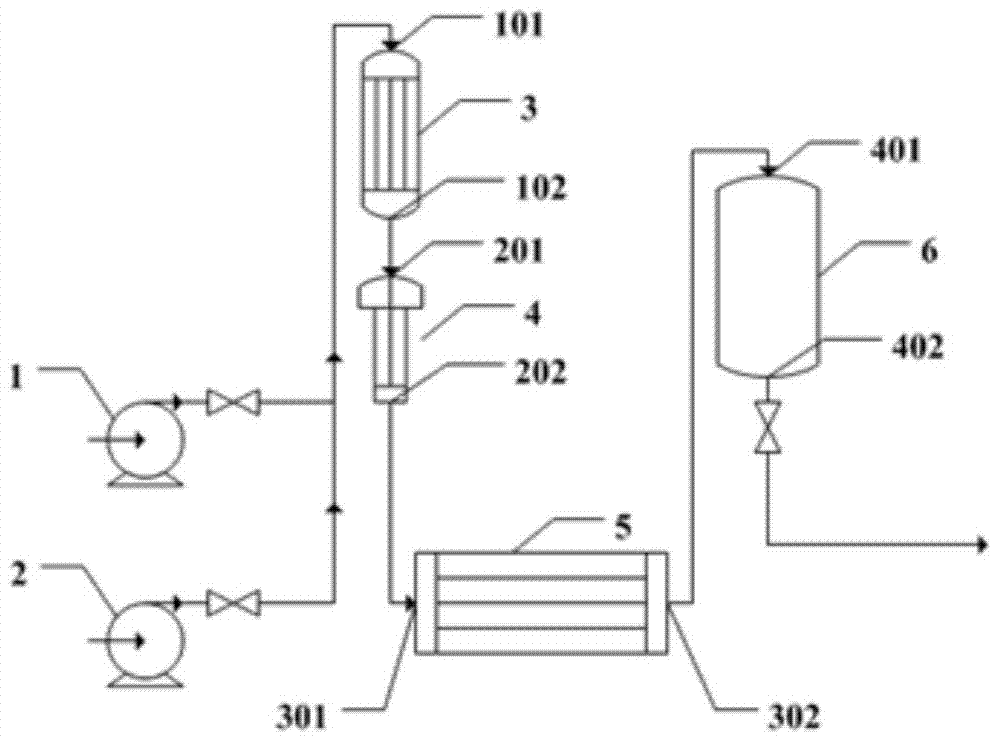
Method and device for continuous preparation of Vildagliptin by tubular reaction
ActiveCN104311467AQuick responseIncrease production capacityOrganic chemistryReaction temperaturePyrrolidine
Owner:SHANGHAI PHARMA GRP QINGDAO GROWFUL PHARMA CO LTD
Process for synthesizing trans-4-amino-1-adamantanol hydrochloride
InactiveCN101735080AThe operating production environment is non-toxicReduce manufacturing costOrganic compound preparationRaney catalystsPatent literatureHydroxylamine Hydrochloride
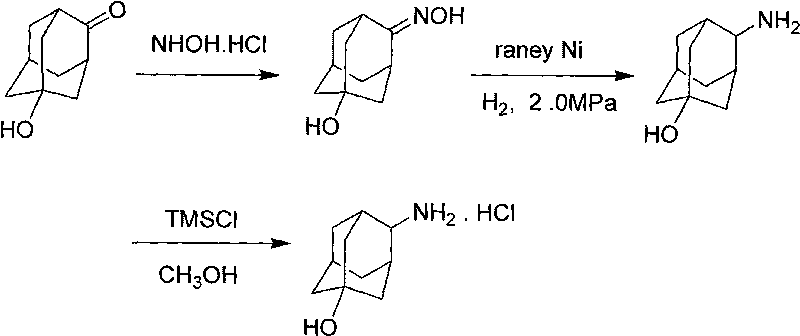
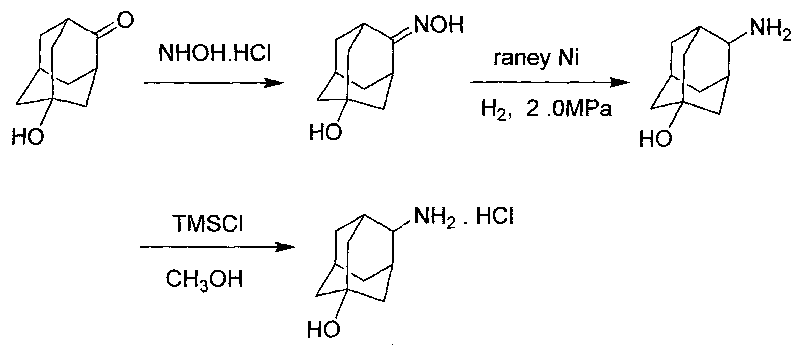
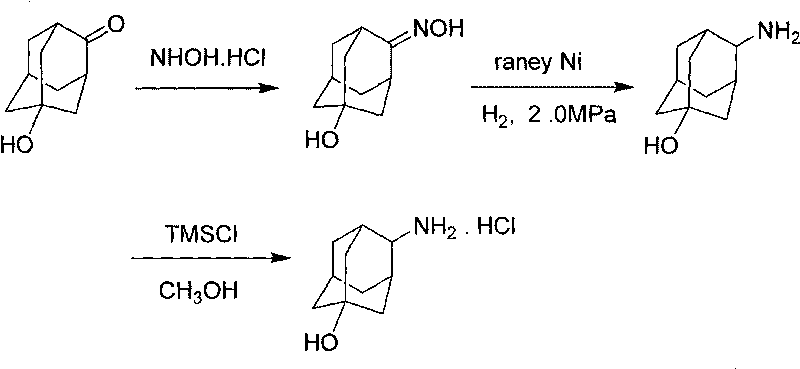
The invention discloses a process for synthesizing trans-4-amino-1-adamantanol hydrochloride, which comprises the steps of taking 5-hydroxy-2-adamantantanone as raw material, oximating by using hydroxylamine hydrochloride, carrying out hydrogenation reduction by using raney nickel for obtaining 4-amino-1-adamantanol, and carrying out three-step reaction of acidification, salt formation and then recrystallization with methanol for obtaining the trans-4-amino-1-adamantanol hydrochloride. Compared with the process reported by the existing patent literature, the process has the advantages of simple operation, easy obtainment of raw materials, low cost, high yield and the like.
Owner:重庆浩康医药化工集团有限公司
Preparation method of isomer impurities of vildagliptin
InactiveCN107311907AQuality improvementHigh purityOrganic chemistryChemical industryTrifluoroacetic anhydride
The invention discloses a preparation method of isomer impurities of vildagliptin, and relates to the technical fields of medicine and chemical industry. The preparation method comprises the steps: S1: enabling D-prolinamide (I) serving as a raw material to react with chloroacetyl chloride to obtain a reaction product (II), and then enabling the reaction product (II) to react with trifluoroacetic anhydride to obtain a reaction product (III); S2: enabling the reaction product (III) to react with 3-amino-1-adamantanol to generate a target product (IV). The preparation method disclosed by the invention has the advantages that the preparation process is simple, the operation is simple and convenient, reaction time is short, and the post-treatment purification is simple and effective, which is beneficial to industrialized production. The purity of manufactured impurities of the vildagliptin is high and is as high as 99.1% through HPLC (high-performance liquid chromatography) detection. By further studying the isomer impurities of the vildagliptin, the quality of the vildagliptin can be better controlled, and the drug safety is improved.
Owner:合肥创新医药技术有限公司
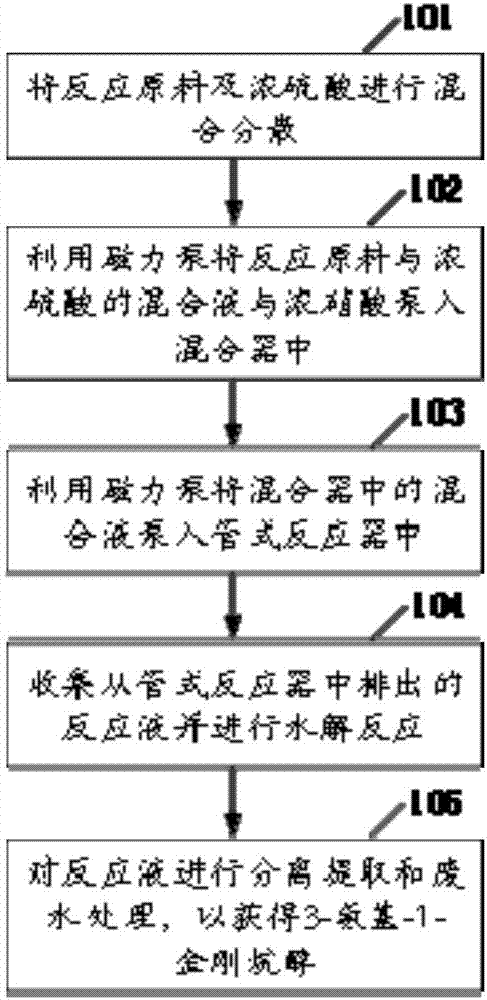
Safe preparation method for adamantanol and device
InactiveCN107325010AReduce processing costsReduce generationOrganic compound preparationAmino-hyroxy compound preparation1-adamantanolDrugs synthesis
The invention relates to the field of drug synthesis and discloses a safe preparation method for adamantanol and a device. The method comprises the following steps: mixing and dispersing reaction raw materials and concentrated sulfuric acid; utilizing a magnetic pump to pump a mixed solution of the reaction raw materials and concentrated sulfuric acid and the concentrated nitric acid into a mixer; utilizing the magnetic pump to pump the mixed solution in the mixer into a tubular reactor; collecting the reaction liquor discharged from the tubular reactor and performing hydrolysis reaction; and separating and extracting the reaction liquor and treating the wastewater, thereby acquiring 3-amino-1-adamantanol. The invention has the advantages that few impurities are generated, the yield of target products is high, the reaction process is safe, the wastewater contains few impurities and the wastewater treating cost is greatly lowered.
Owner:四川众邦新材料股份有限公司
Method for synthesizing 3-amino-1-adamantanol
InactiveCN101747212AEasy to operateHigh yieldOrganic compound preparationAmino-hyroxy compound preparationBromateCurtius rearrangement
The invention discloses a method for synthesizing 3-amino-1-adamantanol. The structural formula of the 3-amino-1-adamantanol is as follows. Using adamantanecarboxylic acid as starting material, the method synthesizes the 3-amino-1-adamantanol according to the following reaction steps: bromate 3-amino-1-adamantanol is synthesized by way of bromination, modified curtius rearrangement and hydrolyzation, and finally, bromate is removed by a alkaline liquor treatment method, so that the 3-amino-1-adamantanol is obtained. The invention has the advantages of simple and easy operation, available materials, less reaction steps and high product yield.
Owner:GUANGDONG UNIV OF TECH
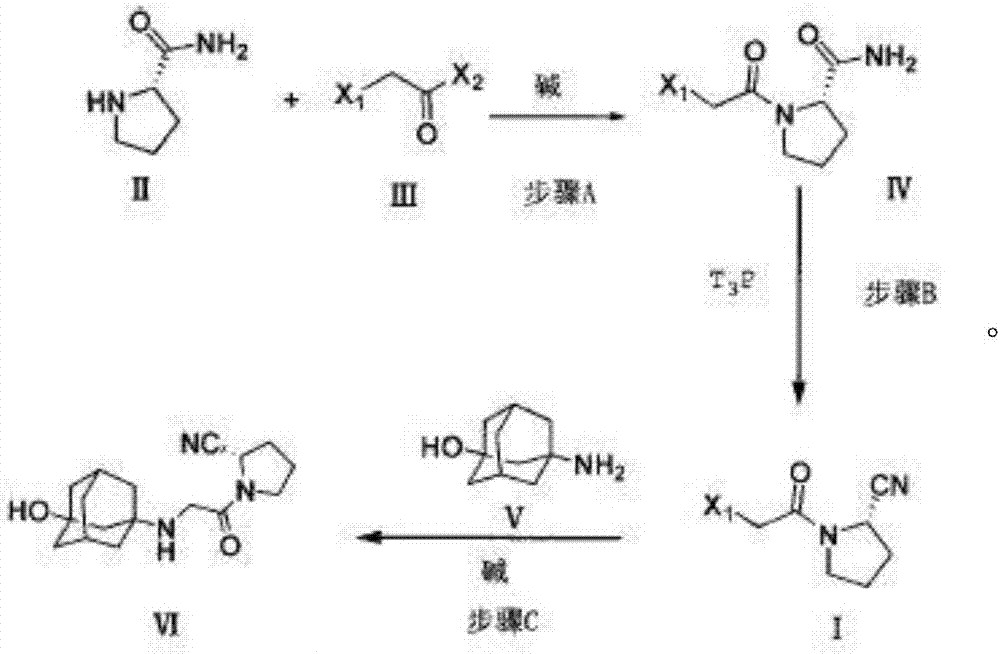
Preparing method for vildagliptin
The invention relates to a preparing method for vildagliptin and belongs to the field of compound preparation. The method comprises the following steps that under an amide organic solvent system and the existence of alkali, 3-amino-1-adamantanol and (S)-1-(2-chloroacetyl)pyrrolidine-2-carbonitrile are subjected to an alkylation reaction, and a reaction solution is obtained; then, the reaction solution is subjected to posttreatment, and vildagliptin is obtained. The method has the advantages that reaction raw materials and a solvent are saved, the yield is high, purity is high, the content of dipolymer impurities is low, the medicinal requirement is met, operation is simple, the reaction time is short, posttreatment is easy, and industrialization is promoted.
Owner:NORTHEAST PHARMA GRP
Process for preparing 3-amino-1-adamantanol
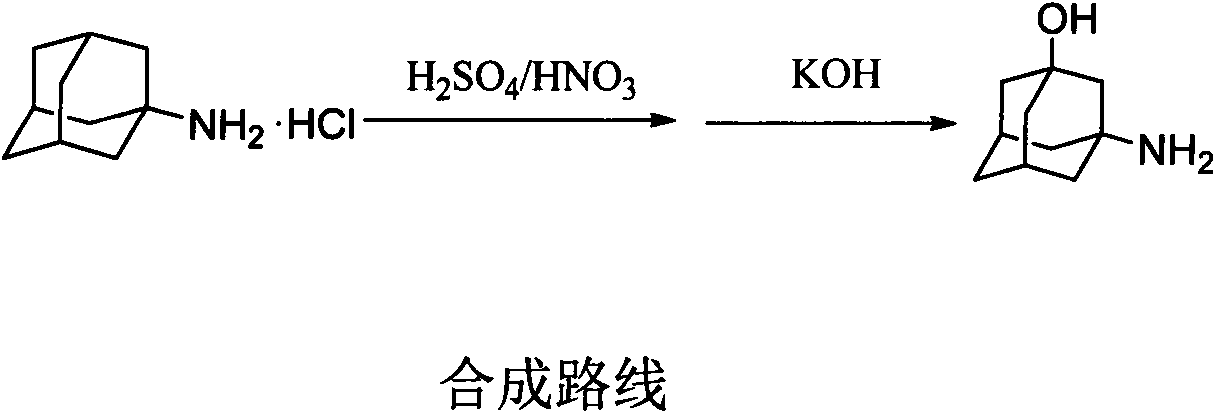
InactiveCN108059601ALow costRaw materials are cheap and easy to getOrganic compound preparationAmino-hyroxy compound preparationAlcoholVildagliptin
The invention discloses a process for preparing a vildagliptin intermediate 3-amino-1-adamantanol. According to the process, amantadine hydrochloride is taken as a starting raw material, and the 3-amino-1-adamantanol is obtained by carrying out a mixed acid reaction on H2SO4 / HNO3, carrying out alkaline hydrolysis on KOH / H2O, and carrying out ethanol extraction. The process is characterized in thatseparation and purification can be performed by using an ethanol extraction process after the alkaline hydrolysis is finished, so that the product yield is greatly increased. The preparation processis easy in obtaining of raw materials, rapid in reaction and high in yield, thus being suitable for industrial production.
Owner:CHONGQING MEDICAL UNIVERSITY
Method for synthesizing 3,5-dibasic-1-adamantine alcohol
A process for synthesizing 3,5-disubstituent-1-adamantanol includes reaction between 1,3-disubstituent adamantane and bromine to obtain bromo compound, adding sodium oxalate and water, reaction, filtering and washing.
Owner:GUANGZHOU INST OF GEOCHEMISTRY - CHINESE ACAD OF SCI
Method for producing 2-adamantanone
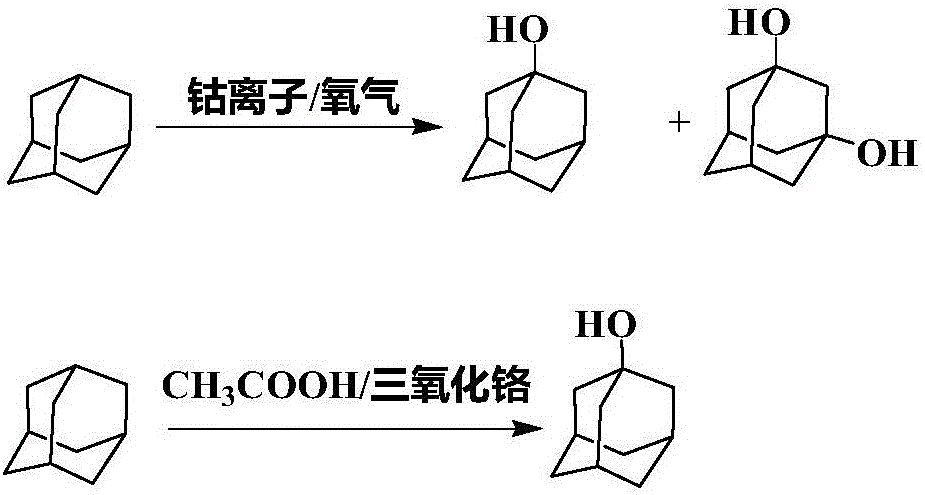
InactiveCN1980877AEfficient preparationOrganic chemistry methodsCarbonyl compound preparation by oxidationCarboxylic acid1-adamantanol
Disclosed is a method for producing 2-adamantanone by oxidizing at least one selected from adamantane and 1-adamantanol wherein sulfuric acid in combination with a carboxylic acid and / or a sulfonic acid is used as an oxidizing agent. By this method, 2-adamantanone can be selectively and efficiently produced in shorter time with higher yield than the conventional methods by oxidizing adamantane or 1-adamantanol.
Owner:IDEMITSU KOSAN CO LTD
Method for Producing 2-Adamantanol and 2-Adamantanone
InactiveUS20080306306A1Improve reaction speedPreparation by isomerisationOrganic compound preparationPhosphoric acidSolid acid
The present invention is a process for producing 2-adamantanol and 2-adamantanone from 1-adamantanol, by using as a catalyst a substance comprising at least one kind of acid catalyst selected from Lewis acid(s) and solid acid(s) that coexist with at least one kind selected from the group consisting of carboxylic acids, sulfonic acids, and phosphoric acids, and provides a process suitable for mass production of 2-adamantanol and 2-adamantanone selectively with high efficiency without using sulfuric acid as a catalyst, thereby enabling laborsaving in waste acid treatment step and drastic reduction of the reaction time.
Owner:IDEMITSU KOSAN CO LTD
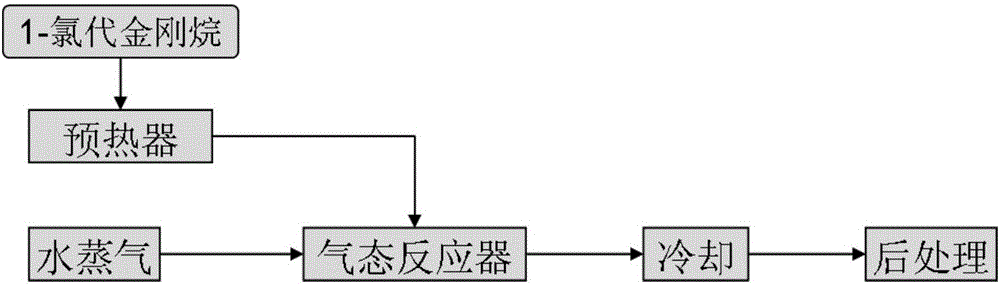
Method for preparing 1-adamantanol
ActiveCN106748643ALow costShort reaction timeOrganic compound preparationHydroxy compound preparation1-adamantanolAdamantane
The invention relates to a method for preparing 1-adamantanol. The method is characterized in that 1-chloroadamantane and vapor react to obtain 1-adamantanol. According to the method, 1-chloroadamantane is taken as the raw material to synthesize 1-adamantanol, the reaction yield is high, the reaction time is short, only one-step operation is required, pollution is small, and a high implementation value is achieved.
Owner:ZHEJIANG NORMAL UNIVERSITY
Method for synthesizing 3-amino-1-adamantanol
InactiveCN101747212BEasy to operateHigh yieldOrganic compound preparationAmino-hyroxy compound preparationBromateCurtius rearrangement
The invention discloses a method for synthesizing 3-amino-1-adamantanol. The structural formula of the 3-amino-1-adamantanol is as follows. Using adamantanecarboxylic acid as starting material, the method synthesizes the 3-amino-1-adamantanol according to the following reaction steps: bromate 3-amino-1-adamantanol is synthesized by way of bromination, modified curtius rearrangement and hydrolyzation, and finally, bromate is removed by a alkaline liquor treatment method, so that the 3-amino-1-adamantanol is obtained. The invention has the advantages of simple and easy operation, available materials, less reaction steps and high product yield.
Owner:GUANGDONG UNIV OF TECH
A kind of preparation method of vildagliptin
The present invention relates to a preparation method of vildagliptin, comprising using L-prolineamide as a raw material, dehydrating with cyanuric chloride to generate (S)-2-cyanopyrrolidine; (S)-2-pyrrolidine The reaction of cyanopyrrolidine and hydrogen chloride to form a salt gives Intermediate ‑1; Intermediate ‑1 reacts with chloroacetic acid under the conditions that EDCI is the condensation agent, HOBt is the catalyst, and DIEA is the acid-binding agent to obtain Intermediate ‑2; Intermediate ‑ 2 react with 3-amino-1-adamantanol to obtain vildagliptin, which is filtered, concentrated, crystallized, and then filtered to obtain the crude product of vildagliptin; after refining with acetone, the finished product of vildagliptin is obtained, and the present invention creates A new route, which can reduce the generation of by-products in each step, reduce the content of by-product vildagliptin disubstituted and 3-amino-1-adamantanol in the product to less than 0.1%, effectively improve the vildagliptin The yield and purity of Tin are high, the raw materials are easy to obtain, and the conditions are mild, so it is suitable for large-scale industrial production.
Owner:HEBEI MEDICAL UNIVERSITY
Preparation method of 1-adamantanol
InactiveCN106631690AHigh selectivitySimple and fast operationOrganic compound preparationHydroxy compound preparationFiltrationDistillation
The invention discloses a preparation method of 1-adamantanol and belongs to the technical field of chemical engineering. The preparation method comprises steps as follows: (1), adamantine is added to a bromine and water mixed system and subjected to heat preservation at three temperature stages in sequence, 1-bromoadamantane is produced through the reaction, and a mixed system containing 1-bromoadamantane is obtained; (2), the mixed system containing 1-bromoadamantane in the step (1) is subjected to atmospheric distillation, excessive bromine is separated out, temperature of the mixed system is controlled, an aqueous solution of sodium metabisulfite is dropwise added, a heat preservation reaction is performed, and a 1-adamantanol crude product is prepared; (3), the 1-adamantanol crude product prepared in the step (2) is added to a mixed solvent prepared from water and an organic solvent mutually soluble with water, activated carbon is added, the mixed solution is heated and filtered, filtrate is crystallized and subjected to suction filtration, and 1-adamantanol is obtained. The preparation method is simple to operate, has low safety risk and few three wastes, and is high in yield and suitable for industrial production.
Owner:VALIANT CO LTD
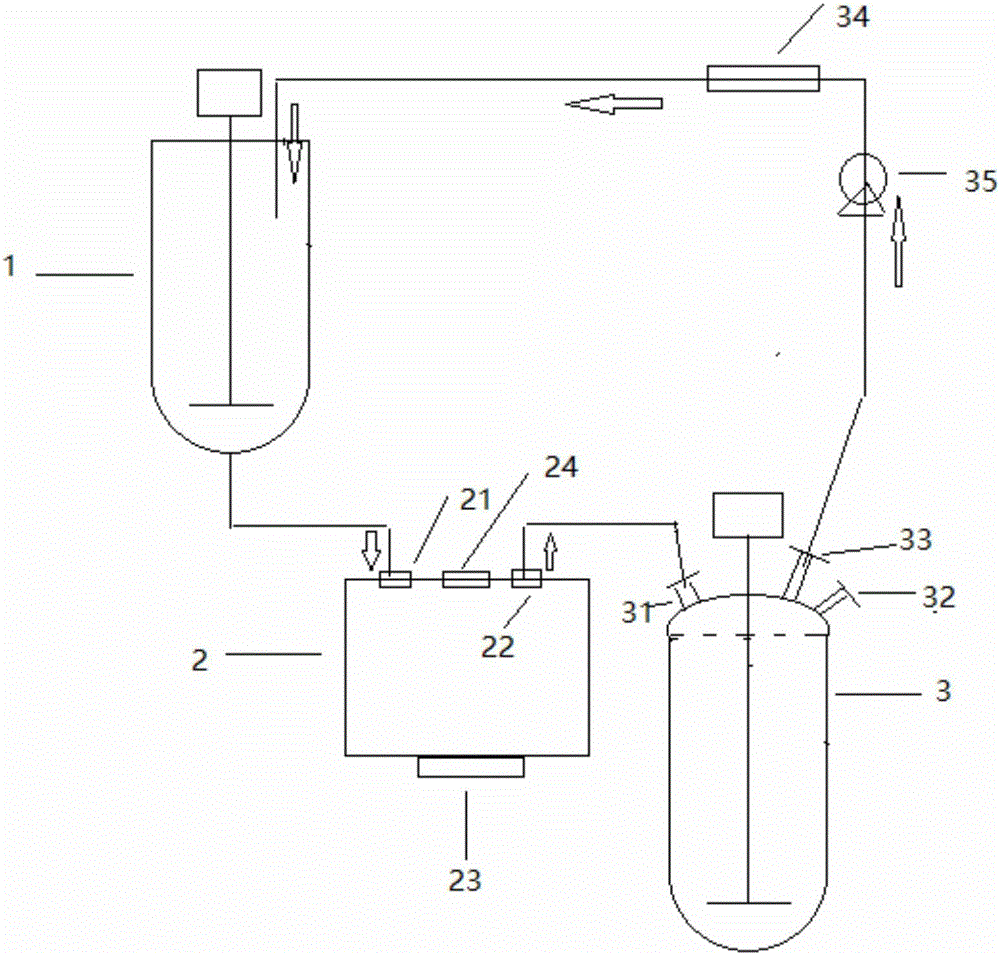
Improved recycling device for producing 3-amino-1-adamantanol
InactiveCN105968020AAvoid wastingAvoid enteringOrganic compound preparationAmino-hyroxy compound preparationChemical industryEngineering
The invention belongs to the field of chemical production, and in particular relates to a recovery and improvement device for producing 3-amino-1-adamantanol, comprising a reaction kettle, a centrifugal device and an extraction kettle; the centrifugal device includes a centrifuge barrel for centrifuging slurry; The upper end of the centrifuge bucket is provided with a centrifugal feed inlet for slurry and water to enter, and a centrifugal outlet for throwing out the discharge liquid when the slurry is centrifuged; the lower end is provided with a discharge outlet for the material generated after the slurry is centrifuged Centrifugal discharge port; the extraction kettle includes a kettle body, the inside of the kettle body is provided with a stirring mechanism, the top of the kettle body is provided with a sealing cover, and an extraction liquid feeding port, an extraction agent feed port, vent valve and extraction discharge port; the centrifugal feed port is connected with the bottom opening of the reaction kettle, and the centrifugal discharge port is connected with the extraction liquid feed port of the extraction kettle ; The extraction outlet is connected with the reaction kettle.
Owner:天津民祥生物医药股份有限公司
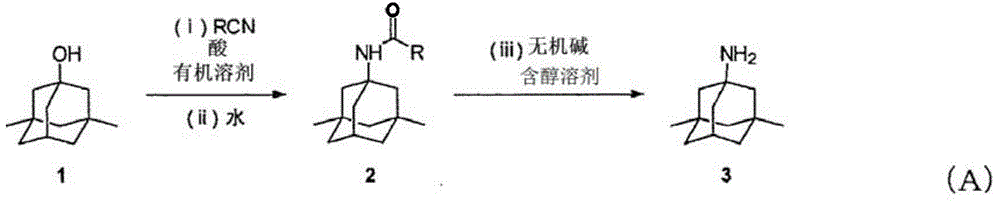
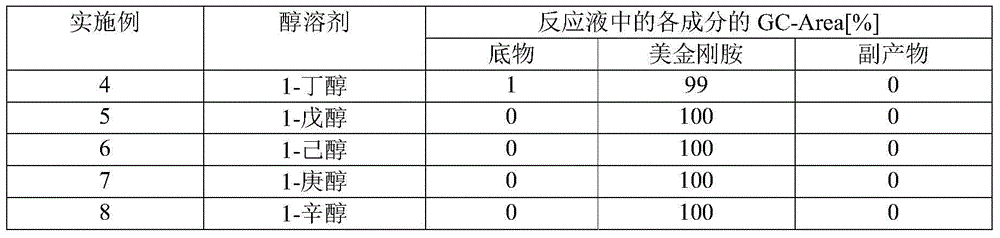
Manufacturing process for memantine
InactiveCN104936942AReduce usageImprove the mixing effectOrganic compound preparationCarboxylic acid amides preparation1-adamantanamineOrganic solvent
This method for manufacturing 3,5-dimethyl-1-adamantanamine includes the following steps (i) through (iii). (i) A step for reacting 3,5-dimethyl-1-adamantanol with oxygen and nitrile in an organic solvent to obtain a reaction solution. (ii) A step for adding water to the reaction solution obtained in step (i) to obtain 1-amido-3,5-dimethyladamantane. (iii) A step for hydrolyzing the 1-amido-3,5-dimethyladamantane obtained in step (ii) in the presence of an alcohol-containing solvent and an inorganic base.
Owner:MITSUBISHI GAS CHEM CO INC
Improvement method for synthesis process of memantine hydrochloride
InactiveCN108218720AReduce dosageReduce volatilityAmino compound purification/separationOrganic compound preparationMemantine HydrochlorideEthane Dichloride
The invention discloses an improvement method for a synthesis process of memantine hydrochloride; with 1,3-dimethyl adamantane as a raw material, 3,5-dimethyl-1-adamantanol is obtained through a bromination reaction and a hydrolysis reaction; 3,5-dimethyl-1-adamantanol is subjected to a chlorination reaction to obtain 1-chloro-3,5-dimethyl adamantane; and 1-chloro-3,5-dimethyl adamantane is subjected to an ammoniation reaction, acid hydrolysis and salt formation, and the target product of methamine hydrochloride is produced, wherein in the bromination reaction, the reaction molar ratio of 1,3-dimethyl adamantane and bromine is 1:1.1, the reaction is performed in dichloroethane, and after the hydrolysis reaction, an organic phase is separated and washed to remove bromine and washed and dried, and an obtained solution is directly used for a next reaction. The improvement method provided by the invention can improve the yield, increase reaction safety and reduce waste and waste water.
Owner:WUJIANG XINKAI MEDICAL TECH
Method for synthesizing 3,5-dibasic-1-adamantine alcohol
Owner:GUANGZHOU INST OF GEOCHEMISTRY - CHINESE ACAD OF SCI
A kind of purification method of vildagliptin crude product
The invention relates to a purification method of a vildagliptin crude product, which is characterized by comprising the following steps: adding a vildagliptin crude product obtained by reacting (S)-1-(2-chloracetyl)pyrryl-2-formonitrile and 3-amino-1-adamantanol disclosed as Formula (II) into a solvent to form a reaction mother solution, adding silica gel into the reaction mother solution, and stirring uniformly at 30-40 DEG C, wherein the solvent is any one of aromatic hydrocarbons, hydrochloric ethers, C1-C5 carboxylates, ketones and alcohols, and the weight ratio of the silica gel to the (S)-1-(2-chloracetyl)pyrryl-2-formonitrile is 0.5:1-6:1; filtering to obtain a filtrate; and finally, recrystallizing to obtain the vildagliptin pure product disclosed as Formula (I), wherein the content of the 3-amino-1-adamantanol raw material disclosed as Formula (II) in the vildagliptin pure product is lower than 0.05%. The purification method can lower the content of the impurity disclosed as Formula (II) to 0.05%, has satisfactory yield, and can implement large-scale application in industry.
Owner:苏州正济药业有限公司
3-amino-1-adamantanol production improvement device
InactiveCN105949068ALow impurity contentReduce the phenomenon of gas-in-liquidOrganic compound preparationAmino-hyroxy compound preparationUltrasound deviceBiochemical engineering
The invention belongs to the field of chemical production, and particularly relates to a 3-amino-1-adamantanol production improvement device. The 3-amino-1-adamantanol production improvement device comprises a reaction liquid storage tank, a magnetic drive pump, and a reaction kettle provided with a stirring paddle, wherein the reaction liquid storage tank, the magnetic drive pump and the reaction kettle are sequentially connected through a liquid delivery tube; an ultrasound device is arranged between the reaction liquid storage tank and the magnetic drive pump and comprises a box body, an ultrasound concussion board arranged in the box body, and an ultrasound generation device arranged outside the box body and connected with the ultrasound concussion board; an inlet end of the box body is connected with the bottom part of the reaction liquid storage tank, and an outlet end of the box body is connected with the magnetic drive pump. The bottom part of the reaction kettle is connected with an inlet of the reaction liquid storage tank. The ultrasound concussion board of the ultrasound device shocks and breaks bubbles so as to greatly reduce the phenomenon of gas-including liquid, the content of the individual impurity of a finished product of 3-amino-1-adamantanol is reduced to be lower than 0.3%, and the content of the product is improved.
Owner:天津民祥生物医药股份有限公司
Trans-4-aminoadamantan-1-ol hydrochloride synthesis technology
InactiveCN105693528AOvercome the problems of many processes and heavy workloadIncrease conversion rate per passOrganic compound preparationAmino-hyroxy compound preparationPatent literatureHydroxylamine Hydrochloride
The invention discloses a trans-4-aminoadamantan-1-ol hydrochloride synthesis technology. The technology comprises that 4-amino-1-adamantanol is prepared from 5-hydroxy-2-adamantanone as a raw material through hydroxylamine hydrochloride oximation and Raney nickel hydrogenation reduction, the 4-amino-1-adamantanol is acidized to form a salt and trans-4-aminoadamantan-1-ol hydrochloride is prepared from the salt through methanol recrystallization three-step reactions. Compared with the prior art reported through the existing patent literature, the technology has the advantages of simple processes, raw material easy acquisition, low cost and high yield.
Owner:QINGDAO SHOUGUAN ENTERPRISE MANAGEMENT CONSULTATION CO LTD
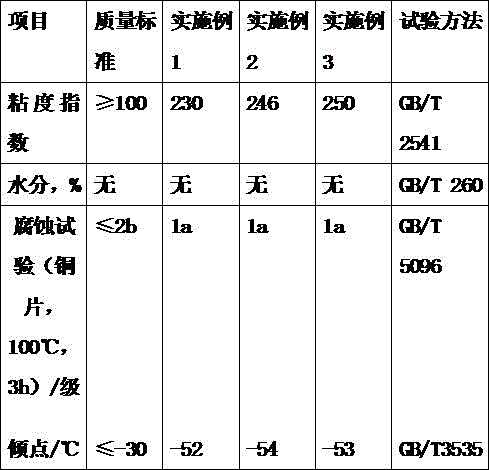
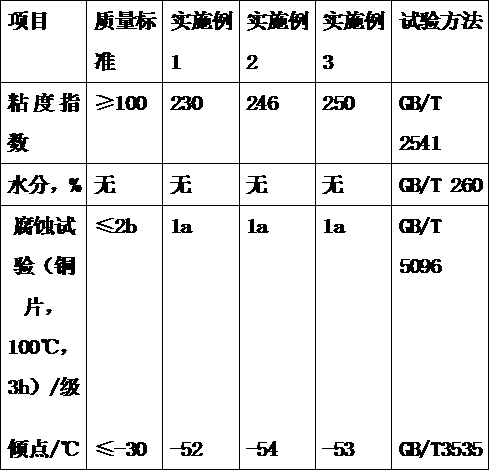
Jack oil used at ultralow temperature
InactiveCN106753718AReduce gelatinImprove low temperature resistanceLubricant compositionAntioxidantTetra
The invention discloses jack oil used at an ultralow temperature. The jack oil is prepared from the following components in parts by weight: 99 parts of hydrogenated tail oil, 0.1-0.3 part of an antirust agent, 0.2-0.4 part of an antioxidant, and 0.5-0.7 part of a functional agent, wherein the functional agent is prepared from the following components in parts by weight: 60-70 parts of 1-adamantanol, 10-12 parts of 3-nonanol, and 6-8 parts of tetra-n-butyl titanate. The jack hydraulic oil prepared by the method provided by the invention can work normally in an ultralow-temperature environment, and greatly improves the performance and the application range of a jack.
Owner:大大科技(宁国)有限公司
A kind of jack oil used at ultra-low temperature
InactiveCN106753718BReduce gelatinImprove low temperature resistanceLubricant compositionAntioxidantEngineering
The invention discloses jack oil used at an ultralow temperature. The jack oil is prepared from the following components in parts by weight: 99 parts of hydrogenated tail oil, 0.1-0.3 part of an antirust agent, 0.2-0.4 part of an antioxidant, and 0.5-0.7 part of a functional agent, wherein the functional agent is prepared from the following components in parts by weight: 60-70 parts of 1-adamantanol, 10-12 parts of 3-nonanol, and 6-8 parts of tetra-n-butyl titanate. The jack hydraulic oil prepared by the method provided by the invention can work normally in an ultralow-temperature environment, and greatly improves the performance and the application range of a jack.
Owner:大大科技(宁国)有限公司
Method and device for continuous preparation of vildagliptin by tubular reaction
Owner:SHANGHAI PHARMA GRP QINGDAO GROWFUL PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com