Synthetic process of vildagliptin
A synthesis technique and technique of the technique are applied in the field of synthesis technique of vildagliptin to achieve the effect of simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
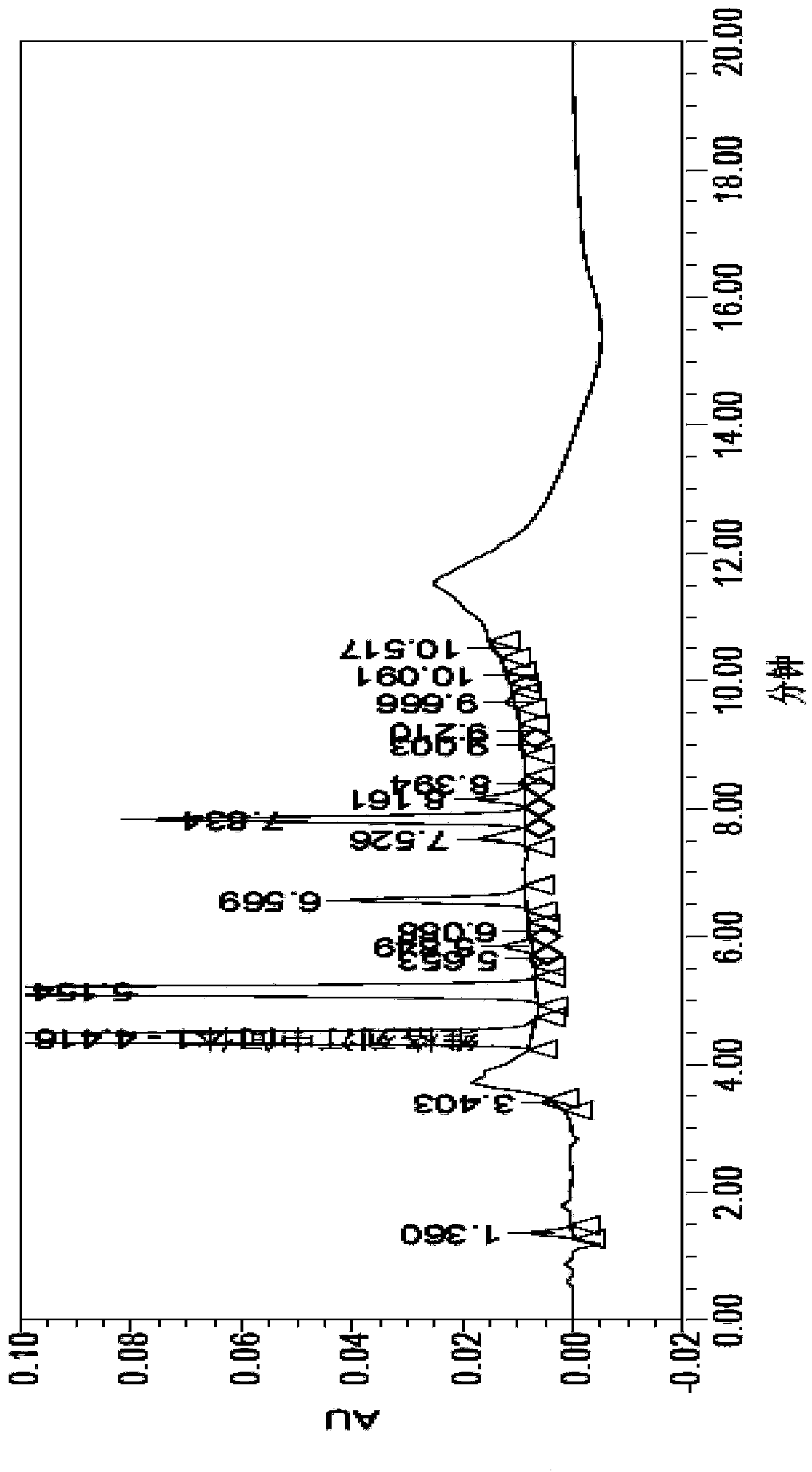
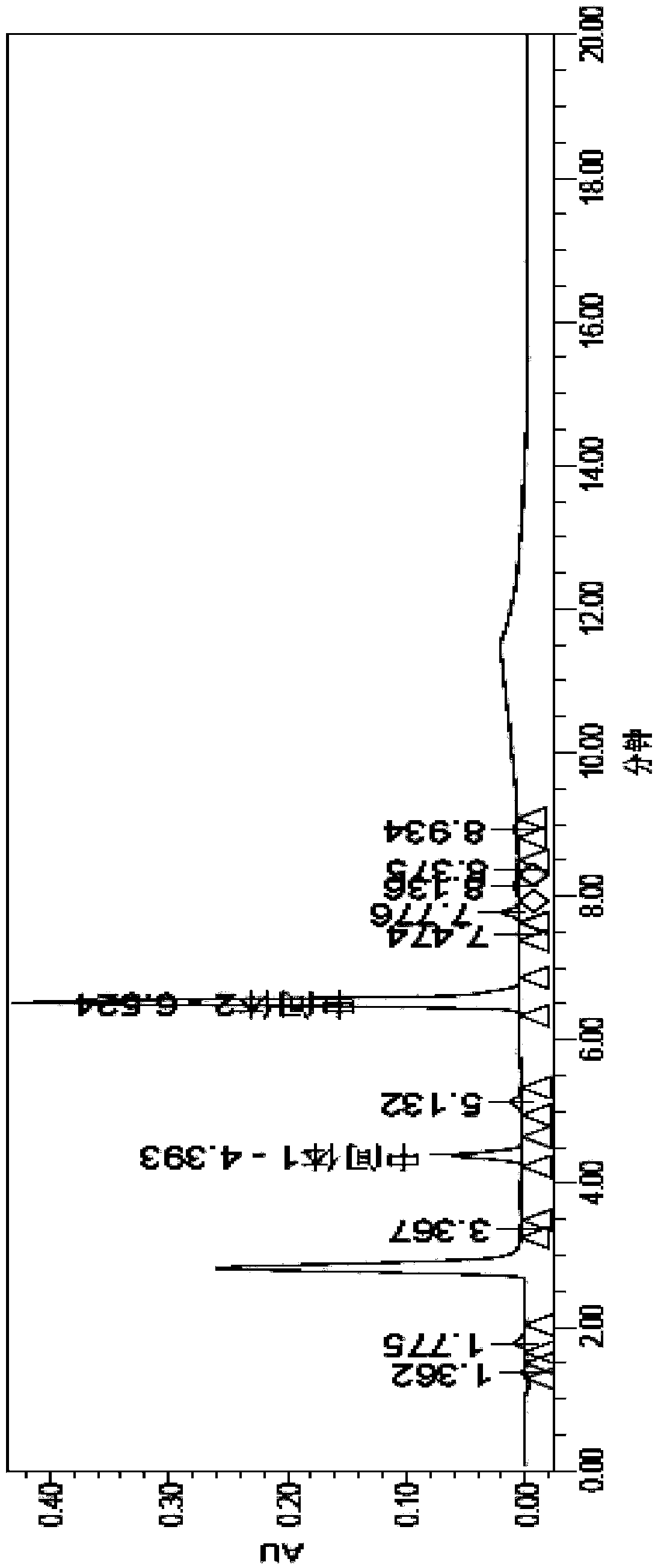
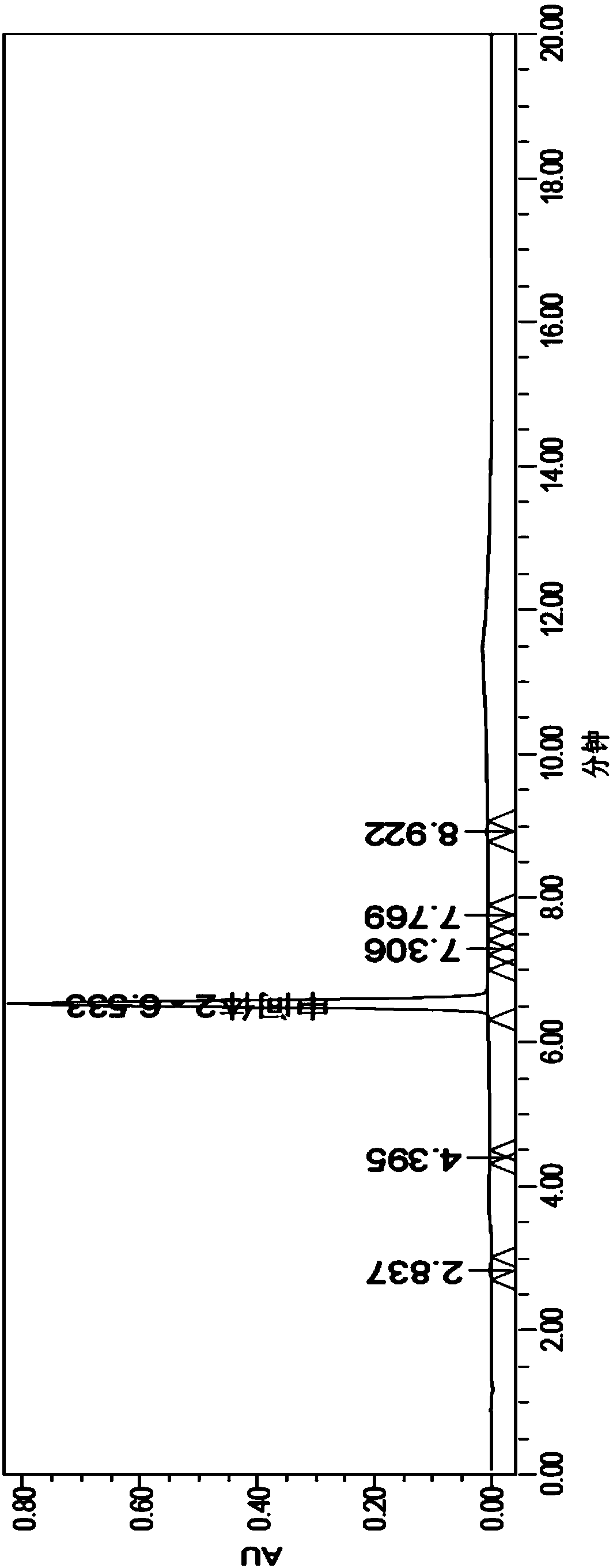
Image
Examples
Embodiment 1
[0081] Embodiment 1, the synthetic method of vildagliptin of the present invention
[0082] (1) (S)-2-cyanopyrrolidine
[0083] Add 2kg of N-fluorenylmethoxycarbonyl-prolinamide to a 50L three-neck reaction flask, add 20L of dichloromethane, stir evenly and cool the system down to -5~0℃, add 2LN,N-dimethylformamide, the system Control the temperature at 0-8°C, and add 1.87kg of trifluoroacetic anhydride dropwise. After the dropwise addition, continue to stir and react for 1.5-2.0 hours. At the same time, set the temperature to 25°C, and the system will heat up naturally. Liquid Phase Detection After the reaction, the system was cooled down to -5°C to 5°C, 8.0L of purified water was added dropwise to the system, allowed to stand for 15 minutes, separated, and the organic phase was washed with purified water (8.0L x 2 times). Transfer the organic phase to a 50L reaction kettle, add 12L of purified water, stir and cool down to 0-10°C, and add 2.0kg of solid sodium bicarbonate to...
Embodiment 2
[0091] Embodiment 2, the existing synthetic method of vildagliptin intermediate II
[0092] figure 2 The synthetic method of described vildagliptin intermediate II: 20g chloroacetyl chloride and 97g potassium carbonate are dissolved in 250mL tetrahydrofuran, mechanically stirred, dropwise add the L-proline amide (20g) solution that is dissolved in tetrahydrofuran (500mL), About 45min, after the dropwise addition, at room temperature, continue to stir the reaction for 2h, filter the reaction solution, remove the potassium salt, and then use Na 2 SO 4 Dry, filter out Na 2 SO 4 Finally, vildagliptin intermediate I was obtained; 25 mL of trifluoroacetic anhydride was added to the transparent filtrate, and magnetically stirred at room temperature for 1 h to obtain a yellow solution, and the excess trifluoroacetic anhydride was removed with ethyl acetate, and the oil was separated and taken The organic layer was washed with Mg 2 SO 4 Dry and filter to obtain intermediate II(s...
Embodiment 3
[0093] Embodiment 3, detection process of the present invention
[0094] The detection process of purity and impurity of vildagliptin final product vildagliptin described in the present invention:
[0095] Chromatographic conditions: use octadecylsilane bonded silica gel as filler (4.6×50μm), use phosphate buffer solution [take 1.3g of anhydrous potassium dihydrogen phosphate solution, put it in a 1000mL volumetric flask, add water to dissolve and dilute to the mark , shake well, adjust the pH to 6.50±0.50 with dipotassium hydrogen phosphate solution (take 1.5g of anhydrous dipotassium hydrogen phosphate, add 10mL of water to dissolve)-water-acetonitrile-methanol (400:600:15:15) as the flow Phase A, phosphate buffer-acetonitrile-methanol (400:450:150) was used as mobile phase B, and the gradient elution was performed according to the following table: the flow rate was 1.8mL / min, the column temperature was 35°C, and the detection wavelength was 210nm. Take vildagliptin referen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com