Improvement method for synthesis process of memantine hydrochloride
A technology of memantine hydrochloride and synthesis process, which is applied in chemical instruments and methods, preparation of amino compounds, preparation of hydroxyl compounds, etc. It can solve the problems of low safety, large amount of bromine, and low yield of concentrated hydrochloric acid, etc., and achieve the reduction of bromine Element volatilization, simplified operation, and simple post-processing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
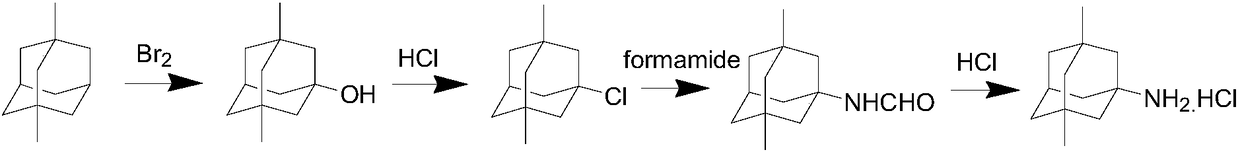
[0018] Take 50g of 1,3-dimethyladamantane into a three-necked flask, add 100ml of 1,2-dichloroethane, add 53.5g of bromine dropwise at room temperature, and heat the reaction solution to 40°C for 2 hours after the dropwise addition. Add 80ml of water at one time, heat to 80°C for an hour, cool to room temperature, separate the phases, wash the organic phase with 5% sodium bisulfite, wash with saturated sodium chloride solution, dry, and filter to obtain 3,5-dimethyl-1 -Adamantanol in 1,2-dichloroethane.
[0019] Add 54g of thionyl chloride to the solution of 3,5-dimethyl-1-adamantanol and 1,2-dichloroethane prepared above, heat to 50°C for 2 hours, cool to room temperature, add 200ml In water, the phases were separated, and the organic phase was washed with 100ml of water, dried, and concentrated to give 54g of a light yellow oily product 1-chloro-3,5-dimethyladamantane, with a yield of 90% and a GC purity of 99.8%
[0020] Take 50g of 1-chloro-3,5-dimethyladamantane into a t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com