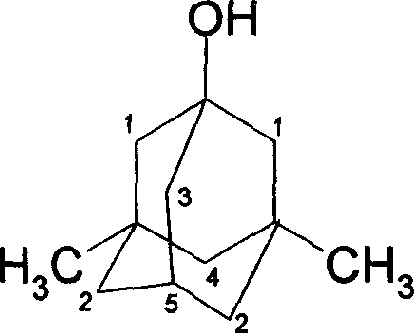
Method for synthesizing 3,5-dibasic-1-adamantine alcohol
A technology of adamantanol and a synthetic method, applied in 3 fields, can solve the problems such as high price of DDQ oxidant, unsatisfactory purification and yield, and yield of only 86%, and achieves low cost, short reaction time, and solvent saving. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Weigh 6.20g (0.0455mol) of adamantane into a 250ml three-neck round bottom flask, stir, add 18ml (0.351mol) of bromine dropwise, and react at 60°C for 2 hours; add 25.0g (0.187mol) to the above reaction solution Sodium oxalate and 55ml of water were reacted at 75°C for 40 minutes, cooled, filtered with suction, and washed with water to obtain 6.86g of 1-adamantanol, yield: 99.05%. mp: 278~280℃; MS: m / z: 152(M + ), 95; 1 H NMR (400Hz, CDCl 3 )2.137 (3H, -CH), 1.60-1.715 (10H, -CH 2 ), 1.70 (1H, -OH); IR (KBr): 3283, 2954, 2851, 1454, 1353, 1118, 1089.
Embodiment 2
[0021] Weigh 6.20g (0.0455mol) of adamantane into a 250ml three-neck round bottom flask, stir, add 12ml (0.234mol) of bromine dropwise, and react at 35°C for 3 hours; add 13.3g (0.099mol) to the above reaction solution Sodium oxalate and 70ml of water were reacted at 55°C for 110 minutes, cooled, suction filtered and washed with water to obtain 6.65g of 1-adamantanol, yield: 96.02%.
Embodiment 3
[0023] Weigh 6.20g (0.0455mol) of adamantane into a 250ml three-neck round bottom flask, stir, add 24ml (0.468mol) of bromine, and react at 70°C for 1 hour; add 36.6g (0.273mol) of oxalic acid to the above reaction solution Sodium and 80ml of water were reacted at 80°C for 25 minutes, cooled, filtered with suction, and washed with water to obtain 6.88g of 1-adamantanol, yield: 99.35%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com