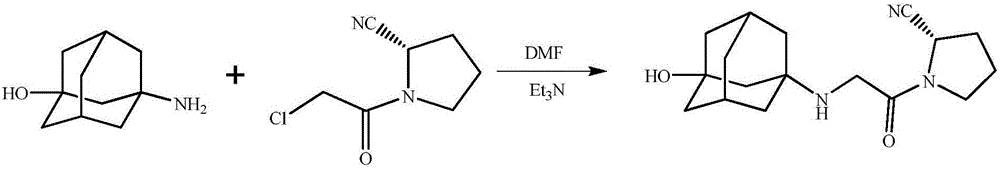
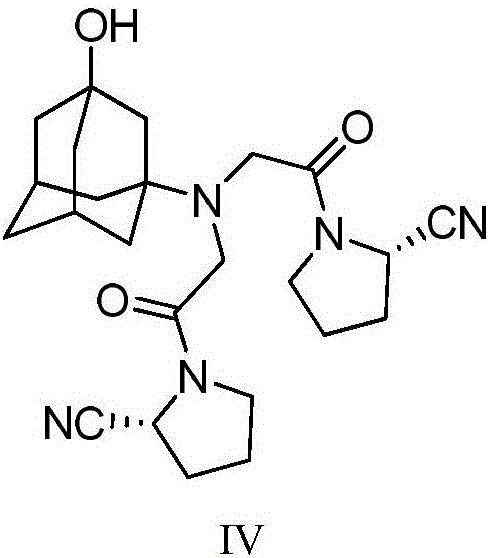
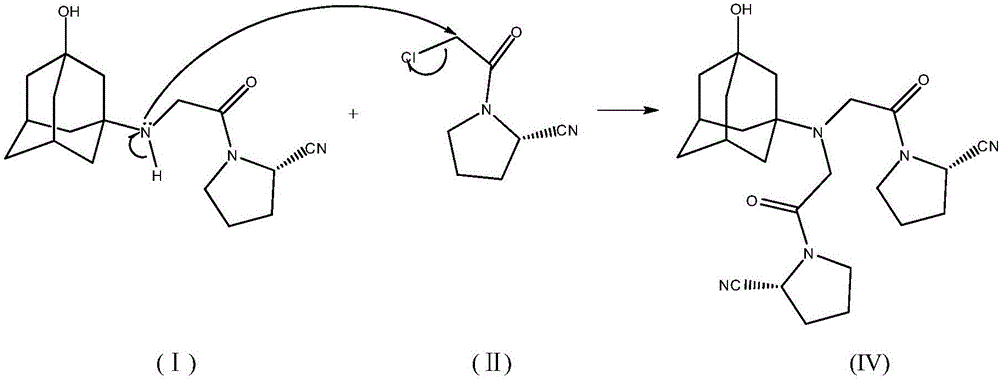
Preparing method for vildagliptin
A technology of amides and organic solvents, applied in the field of preparation of medicinal compounds, to achieve the effects of saving reaction raw materials and solvents, high purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The impact of different molar ratios of raw materials on the reaction yield:
[0025] (1) Add 80.0g N,N-dimethylformamide, (S)-1-(2-chloroacetyl)pyrrolidine-2-carbonitrile, 3-amino-1-adamantanol to a 250mL reaction flask , triethylamine, the selection of different molar ratios of the raw materials is shown in Table 1, and the reaction solution was obtained by stirring and reacting at 20° C. to 30° C. for 20 hours;
[0026] (2) Extraction: Distill the reaction solution under reduced pressure at 60-70°C, lower the temperature to below 30°C, add 120.0g of dichloromethane and 60.0g of half-saturated aqueous ammonium chloride solution, add 40ml of saturated aqueous sodium bicarbonate solution, and stir The layers were placed, and the dichloromethane layer was washed once with 50.0 g of water to obtain an organic phase extract;
[0027] (3) Drying: Add 8g of anhydrous magnesium sulfate to the organic phase extract and dry for 1-2 hours, filter, concentrate under reduced pres...
Embodiment 2
[0032] The influence of adopting different organic bases on the reaction yield:
[0033] (1) Add 80.0g N,N-dimethylformamide, 13.8g (0.08mmol) (S)-1-(2-chloroacetyl)pyrrolidine-2-carbonitrile, 13.4g to a 250mL reaction flask (0.08mmol) 3-amino-1-adamantanol, 0.08mmol organic base, the selection of the organic base is shown in Table 2, and it was incubated at 20°C to 30°C and stirred for 20 hours to obtain a reaction solution;
[0034] (2) Extraction: Distill the reaction solution under reduced pressure at 60-70°C, lower the temperature to below 30°C, add 120.0g of dichloromethane and 60.0g of half-saturated aqueous ammonium chloride solution, add 40ml of saturated aqueous sodium bicarbonate solution, and stir The layers were placed, and the dichloromethane layer was washed once with 50.0 g of water to obtain an organic phase extract;
[0035] (3) Drying: Add 8g of anhydrous magnesium sulfate to the organic phase extract and dry for 1-2 hours, filter, concentrate under reduced...
Embodiment 3
[0040] The influence of adopting different organic solvents on the reaction yield:
[0041] (1) Add 80.0g organic solvent, 13.8g (0.08mmol) (S)-1-(2-chloroacetyl)pyrrolidine-2-carbonitrile, 13.4g (0.08mmol) 3-amino - 1-adamantanol, 8.1g (0.08mmol) triethylamine, see Table 3 for the selection of the organic solvent, keep warm at 20°C to 30°C and stir for 20 hours to obtain a reaction solution;
[0042] (2) Extraction: Distill the reaction solution under reduced pressure at 60-70°C, lower the temperature to below 30°C, add 120.0g of dichloromethane and 60.0g of half-saturated aqueous ammonium chloride solution, add 40ml of saturated aqueous sodium bicarbonate solution, and stir The layers were placed, and the dichloromethane layer was washed once with 50.0 g of water to obtain an organic phase extract;
[0043] (3) Drying: Add 8g of anhydrous magnesium sulfate to the organic phase extract and dry for 1-2 hours, filter, concentrate under reduced pressure, and concentrate until n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com