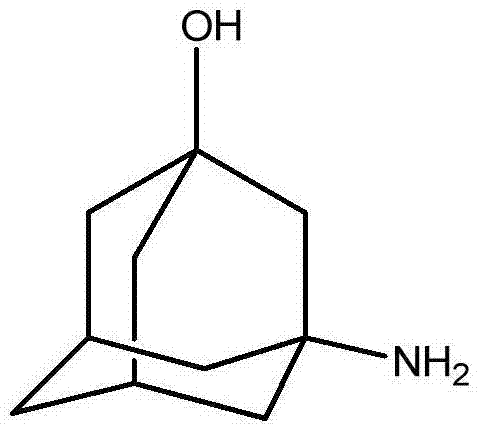
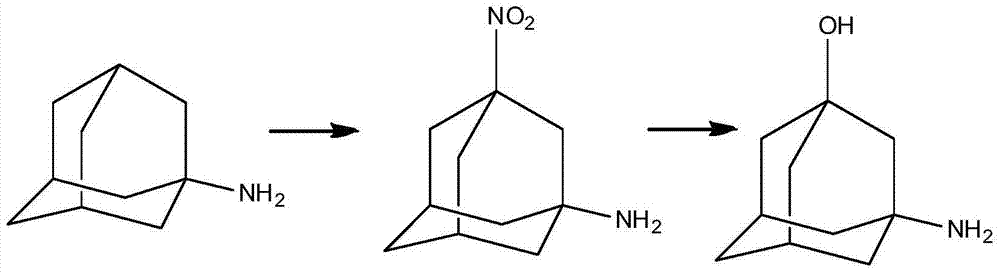
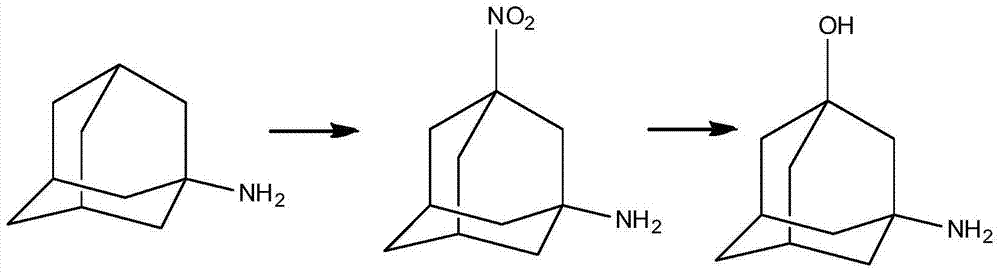
Preparation method of 3-amino-1-adamantanol
A technology for adamantane alcohol and amantadine amine, which is applied in the field of preparation of 3-amino-1-adamantanyl alcohol, can solve the problems of complicated operation, unsuitable for industrial production and high cost, and achieves simple operation, environmental friendliness and low cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Preparation of mixed acid: under normal temperature, add 96.9g (1mol) of concentrated nitric acid dropwise into 735.6g (7.5mol) of concentrated sulfuric acid, keeping the temperature not higher than 30°C. In the mixed acid, the molar ratio of concentrated nitric acid to concentrated sulfuric acid is 1:7.5.
[0044] (1) Under normal temperature conditions, 735.6g (7.5mol) concentrated sulfuric acid is added in the reactor, then add 187.7g (1mol) amantadine hydrochloride in batches, stir, until dissolving, amantadine hydrochloride and The molar ratio of concentrated sulfuric acid is 1:7.5. Add the mixed acid dropwise to the reactor, keeping the temperature not higher than 30°C. When 1 / 3 of the amount is added dropwise, stir for about 1 hour, then add the remaining mixed acid dropwise. After the dropwise addition, the amantadine hydrochloride in the reactor The molar ratio of salt to concentrated nitric acid is 1:1. After stirring at room temperature for 16 hours, GC (ga...
Embodiment 2
[0050] Preparation of mixed acid: Add 96.9g (1mol) of concentrated nitric acid dropwise into 441.4g (4.5mol) of concentrated sulfuric acid at room temperature, keeping the temperature not higher than 30°C. In the mixed acid, the molar ratio of concentrated nitric acid to concentrated sulfuric acid is 1:4.5.
[0051] (1) Under normal temperature conditions, add 441.4g (4.5mol) of concentrated sulfuric acid into the reactor, then add 187.7g (1mol) of amantadine hydrochloride in batches, stir to obtain a white turbid liquid, amantadine hydrochloride The molar ratio to concentrated sulfuric acid is 1:4.5. Add the mixed acid dropwise into the reactor and keep the temperature not higher than 30°C. When 1 / 3 of the amount is added dropwise, stir for 1 hour, then add the remaining mixed acid dropwise. After the dropwise addition, amantadine hydrochloride and The molar ratio of concentrated nitric acid is 1:1. After stirring at room temperature for 10 hours, the GC central control sho...
Embodiment 3
[0054] Preparation of mixed acid: Add 126.0g (2.0mol) of fuming nitric acid dropwise into 490.4g (5.0mol) of concentrated sulfuric acid at room temperature, keeping the temperature not higher than 30°C. In the mixed acid, the molar ratio of fuming nitric acid to concentrated sulfuric acid is 1:2.5.
[0055] (1) Under normal temperature conditions, add 490.4g (5.0mol) of concentrated sulfuric acid into the reactor, then add 187.7g (1mol) of amantadine hydrochloride in batches, and stir to obtain a white turbid liquid. The molar ratio of amantadine hydrochloride to concentrated sulfuric acid is 1:5.0. Add the mixed acid dropwise into the reactor and keep the temperature not higher than 30°C. When 1 / 3 of the amount is added dropwise, stir for 1 hour, then add the remaining mixed acid dropwise. After the dropwise addition, the molar ratio of amantadine hydrochloride to concentrated nitric acid in the reactor was 1:2. After stirring at room temperature for 18 hours, the GC contro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com