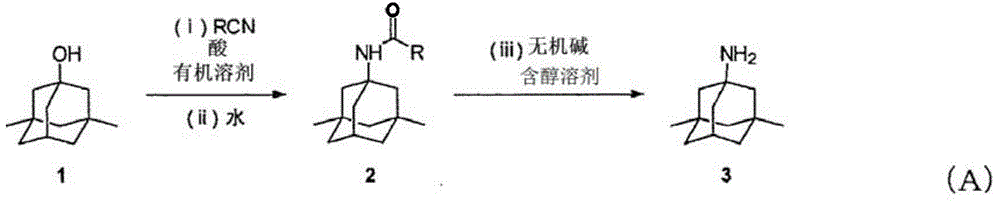
Manufacturing process for memantine
A technology of amantadine and its manufacturing method, which is applied in the direction of preparation of carboxylic acid amide, preparation of organic compounds, preparation of amino compounds, etc., can solve the problems of neuroexcitotoxicity protective effects, etc., and achieve improved stirring performance, reduced usage, cost reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] 3,5-Dimethyl-1-adamantanol: 10.00 g (55.5 mmol), acetonitrile: 4.55 g (110.9 mmol), and toluene: 25.00 g were added to a 300 mL round bottom flask to obtain a mixed solution. In addition, 3,5-dimethyl-1-adamantanol was produced according to the method described in p.7390-7391 of Journal of the American Chemical Society (vol. 122, 30, 2000). Next, 97% concentrated sulfuric acid: 11.00 g (108.8 mmol) was added dropwise to the mixed solution in the flask over 23 minutes, and the resulting reaction solution was stirred at 21° C. for 2 hours to continue the reaction. In the reaction solution, after confirming the disappearance of 3,5-dimethyl-1-adamantanol by gas chromatography (GC), water: 33.66 g was added to the reaction solution to stop the reaction, and 1-acetamide-3, 5-Dimethyladamantane in toluene (2-phase solution). 1-hexanol: 25.01g was added to this two-phase solution, and liquid separation operation was performed twice, and the water phase was removed from the tw...
Embodiment 2
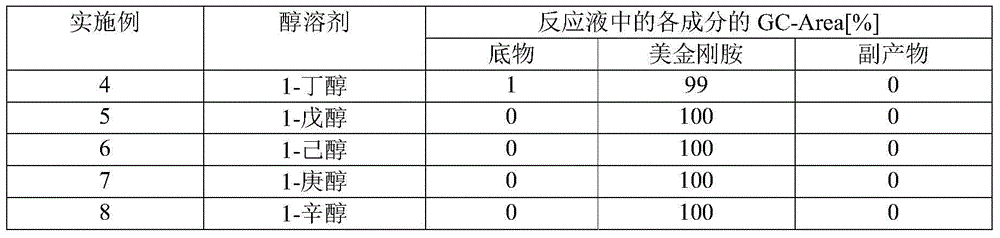
[0105] 3,5-Dimethyl-1-adamantanol: 1.00 g (5.50 mmol), acetonitrile: 0.46 g (11.1 mmol), and mesitylene: 9.61 g were added to a test tube with an outer diameter of 30 mm to obtain a mixed liquid. Then, 97% concentrated sulfuric acid: 1.12 g (11.1 mmol) was added dropwise to the mixed solution in the test tube, and the resulting reaction solution was stirred at 30° C. for 3 hours to continue the reaction. Then, in the reaction solution, add water: 6.09g to stop the reaction, wash and remove the water phase from the reaction solution, thereby obtaining a mesitylene solution containing 1-acetamide-3,5-dimethyladamantane (reaction yield : 80.4%). Then, sodium hydroxide (NaOH): 0.71 g and 1-hexanol: 9.61 g were added to the obtained solution, and the obtained reaction solution was stirred at 130° C. for 18 hours to perform 1-acetamide-3,5 - Hydrolysis of dimethyladamantane. Then, in this reaction solution, formation of memantine was confirmed by GC (reaction yield: 96.2%).
Embodiment 3
[0107] 3,5-Dimethyl-1-adamantanol: 0.09 g (0.50 mmol), acetonitrile: 0.25 g (6.0 mmol), and toluene: 0.87 g were added to a test tube with an outer diameter of 15 mm to obtain a mixed liquid. Then, p-toluenesulfonic acid: 0.19 g (1.00 mmol) was added to the mixed solution in the test tube, and the obtained reaction solution was stirred at 70° C. for 24 hours to continue the reaction. Then, add water: 1.00g to stop the reaction in the reaction solution, wash and remove the water phase from the reaction solution, thus obtaining a toluene solution containing 1-acetamide-3,5-dimethyladamantane (reaction yield: 64 %). Then, sodium hydroxide (NaOH): 0.052 g and 1-hexanol: 0.82 g were added to the obtained solution, and the obtained reaction solution was stirred at 126° C. for 18 hours to perform 1-acetamide-3,5 - Hydrolysis of dimethyladamantane. Then, in this reaction solution, formation of memantine was confirmed by GC (reaction yield: 96%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com