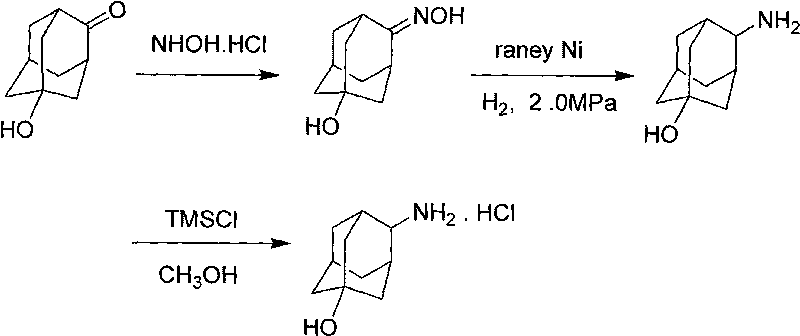
Process for synthesizing trans-4-amino-1-adamantanol hydrochloride
A kind of technology of adamantanol and synthesis process, which is applied in the preparation of amino hydroxyl compounds, the preparation of organic compounds, Raney type catalysts, etc., can solve the problems of expensive, difficult to prepare, and unsuitable for industrial production, and achieve low price and high product quality. The effect of high quality and yield and reduction of production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The synthetic route of the present invention is as follows:
[0027]
[0028] Preparation of 5-Hydroxy-2-adamantanone oxime
[0029] 5-Hydroxy-2-adamantanone (15g, 90.2mmol) was dissolved in 100ml of ethanol, 80ml of 1N sodium hydroxide solution of hydroxylamine hydrochloride (10g, 143.9mmol) was added, and the temperature was raised to 100°C for 2 hours. Ethanol was evaporated, water and dichloromethane were added, stirred for 10 minutes, filtered, the solid was collected, the filtrate was separated, the water phase was extracted twice with dichloromethane, the organic phase was combined and the solvent was distilled off, the obtained solid was combined with the filter cake, Ethyl acetate was recrystallized to obtain 5-hydroxy-2-adamantanone oxime (12 g, mass yield 80%).
[0030] Preparation of 4-amino-1 adamantanol
[0031] Add 5-hydroxy-2-adamantanone oxime (10g, 55mmol), 100ml ethanol, 2g Raney nickel into the autoclave, after nitrogen replacement, adjust the h...
Embodiment 2
[0035] Preparation of 5-Hydroxy-2-adamantanone oxime
[0036] 5-Hydroxy-2-adamantanone (15g, 90.2mmol) was dissolved in 100ml of ethanol, 80ml of 1N sodium hydroxide solution of hydroxylamine hydrochloride (10g, 143.9mmol) was added, and the temperature was raised to 100°C for 2 hours. Ethanol was evaporated, water and dichloromethane were added, stirred for 10 minutes, filtered, the solid was collected, the filtrate was separated, the water phase was extracted twice with dichloromethane, the organic phase was combined and the solvent was distilled off, the obtained solid was combined with the filter cake, Ethyl acetate was recrystallized to obtain 5-hydroxy-2-adamantanone oxime (12 g, mass yield 80%).
[0037] Preparation of 4-amino-1 adamantanol
[0038] Add 5-hydroxyl-2-adamantanone oxime (10g, 55mmol), 100ml alcohol solvent in the autoclave, select one or more of ethanol, methanol, isopropanol, n-butanol, tert-butanol, 0.5g of Raney nickel, after nitrogen replacement, ad...
Embodiment 3
[0042] Preparation of 5-Hydroxy-2-adamantanone oxime
[0043]5-Hydroxy-2-adamantanone (15g, 90.2mmol) was dissolved in 100ml of ethanol, 80ml of 1N sodium hydroxide solution of hydroxylamine hydrochloride (10g, 143.9mmol) was added, and the temperature was raised to 100°C for 2 hours. Ethanol was evaporated, water and dichloromethane were added, stirred for 10 minutes, filtered, the solid was collected, the filtrate was separated, the water phase was extracted twice with dichloromethane, the organic phase was combined and the solvent was distilled off, the obtained solid was combined with the filter cake, Ethyl acetate was recrystallized to obtain 5-hydroxy-2-adamantanone oxime (12 g, mass yield 80%).
[0044] Preparation of 4-amino-1 adamantanol
[0045] Add 5-hydroxy-2-adamantanone oxime (10g, 55mmol), 100ml methanol, 5g Raney nickel into the autoclave, after nitrogen replacement, adjust the hydrogen pressure to 5.0MPa, and react at about 0°C for 48 hours. After filtration...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com