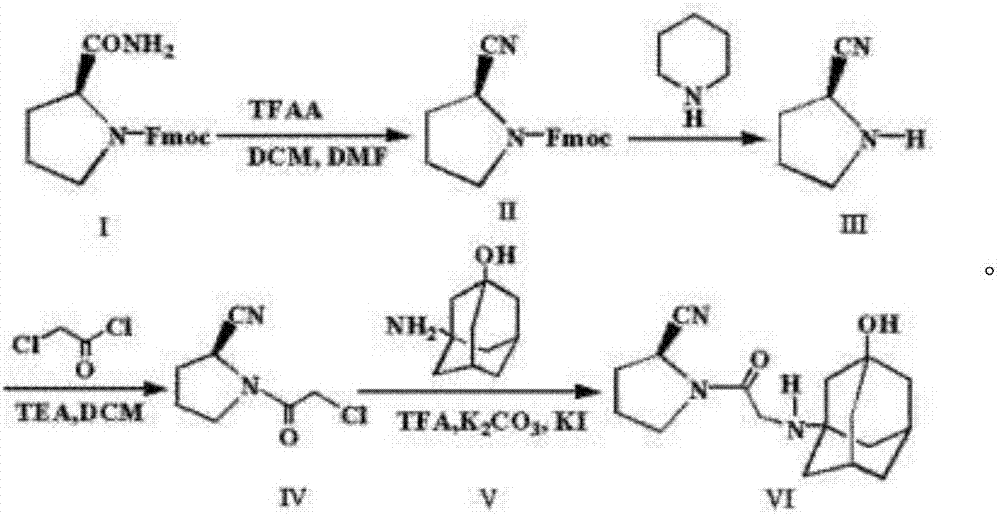
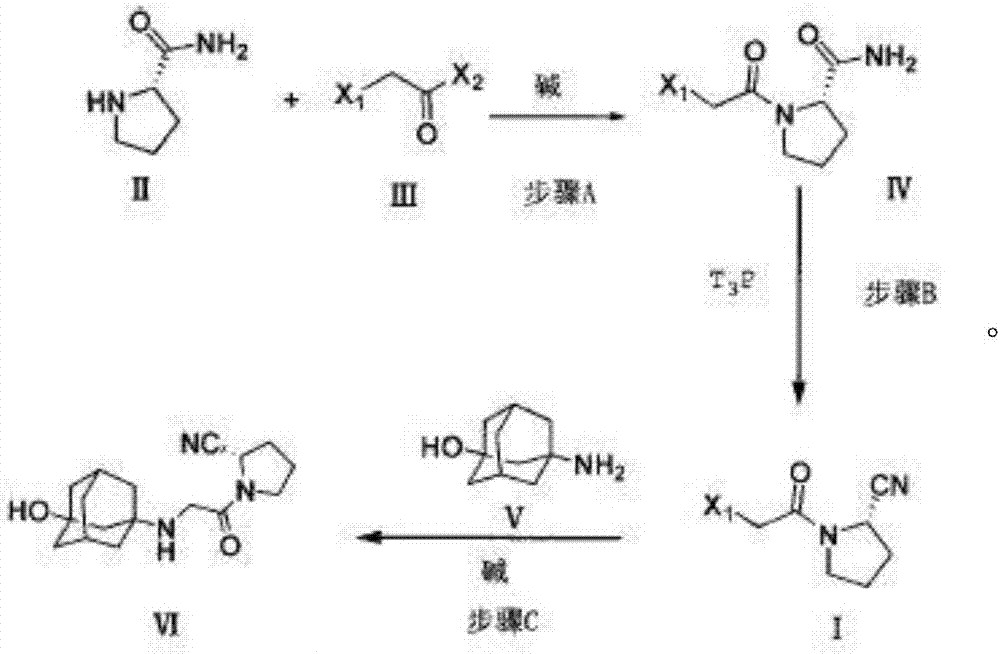
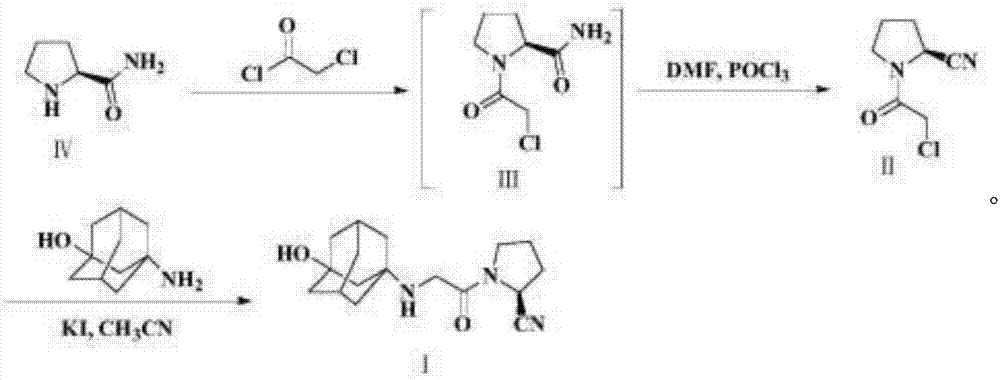
Preparation method for Vildagliptin
A technology of solvent and cyanuric chloride, which is applied in the field of preparation of vildagliptin, can solve the problems that the purity of the product cannot be guaranteed, and the formation of by-products is not easy to control, so as to reduce the content of by-products, reduce energy consumption, and ensure the purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Synthesis of Vildagliptin Intermediate-1
[0053] Add 100g of L-prolineamide and 300ml of N,N-dimethylformamide into a 1L reaction bottle, control the temperature to 0-10°C under stirring, add 80.8g of cyanuric chloride slowly in batches, Insulation reaction 2h. The reaction solution was poured into 600ml of purified water, adjusted to pH 9-10 with saturated aqueous sodium carbonate solution, and extracted three times with ethyl acetate, 600ml each time. Combine the organic phases, wash with 500ml of saturated aqueous sodium bicarbonate solution, add 50g of anhydrous sodium sulfate, and stir at 20-30°C for 1h. Filter, wash the filter cake with 100ml of ethyl acetate, concentrate the filtrate to dryness under reduced pressure to obtain an oil, add 300ml of ethyl acetate, adjust the pH to 2~3 with 30% hydrogen chloride ethanol solution, cool down to 0~10°C, stir and crystallize for 2h . After filtering, the filter cake was washed with 100 ml of ethyl acetate, and the f...
Embodiment 2
[0061] Synthesis of Vildagliptin Intermediate-1
[0062] Add 100g of L-prolineamide and 300ml of N,N-dimethylacetamide into a 1L reaction bottle, control the temperature to 0-10°C under stirring, add 50.9g of cyanuric chloride slowly in batches, Insulation reaction 2h. The reaction solution was poured into 600ml of purified water, adjusted to pH 9-10 with saturated aqueous sodium carbonate solution, and extracted three times with dichloromethane, 600ml each time. Combine the organic phases, wash with 500ml of saturated aqueous sodium bicarbonate solution, add 50g of anhydrous sodium sulfate, and stir at 20-30°C for 1h. Filter, wash the filter cake with 100ml of dichloromethane, concentrate the filtrate to dryness under reduced pressure to obtain an oil, add 300ml of ethyl acetate, adjust the pH to 2~3 with 20% hydrogen chloride ethanol solution, cool down to 0~10°C, stir and crystallize for 2h . After filtering, the filter cake was washed with 100 ml of ethyl acetate, and t...
Embodiment 3
[0070] Synthesis of Vildagliptin Intermediate-1
[0071] Add 100g of L-prolineamide and 300ml of dimethyl sulfoxide into a 1L reaction flask, control the temperature to 0-10°C under stirring, slowly add 80.8g of cyanuric chloride in batches, and keep the reaction at 0-10°C for 2 hours. Pour the reaction solution into 600ml of purified water, adjust the pH to 9-10 with saturated aqueous sodium carbonate solution, and extract with toluene three times, 600ml each time. Combine the organic phases, wash with 500ml of saturated aqueous sodium bicarbonate solution, add 50g of anhydrous sodium sulfate, and stir at 20-30°C for 1h. Filter, wash the filter cake with 100ml of toluene, concentrate the filtrate to dryness under reduced pressure to obtain an oil, add 300ml of toluene, adjust the pH to 2~3 with 40% hydrogen chloride ethanol solution, cool down to 0~10°C, stir and crystallize for 2h. After filtration, the filter cake was washed with 100ml of toluene, and the filter cake was v...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com