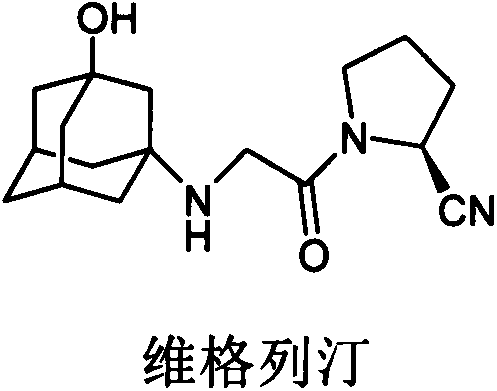
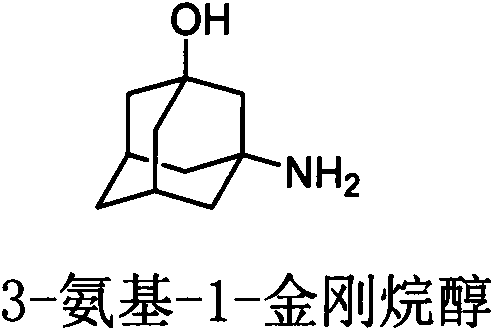
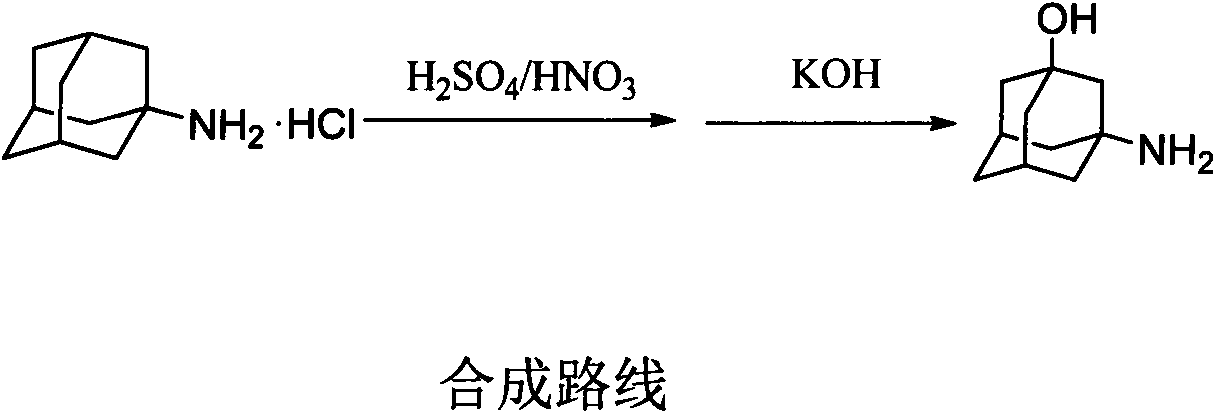
Process for preparing 3-amino-1-adamantanol
A kind of adamantanol and process technology, applied in the field of chemical synthesis preparation, can solve the problems of complicated operation and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]Under ice-bath conditions, add 37.5ml of 98% concentrated sulfuric acid into the round bottom flask, add 3.75ml of 65% concentrated nitric acid under stirring, and continue stirring for 10min. When the temperature of the reaction solution dropped to 0°C, 3.75 g of amantadine hydrochloride was added in portions. After the addition was complete, the reaction was continued for 2 h in the ice bath, and then the ice bath was removed, and the reaction was continued for 2 h at room temperature. Transfer the system to a three-neck flask, add 60g of crushed ice while stirring, until the reaction solution turns clear dark green, put it in an ice bath, add an appropriate amount of potassium hydroxide solid, adjust the pH of the solution to > 12, and keep the solution on ice The reaction was continued for 1 h under bath conditions, producing a large amount of white sticky solid. Suction filtration, collect the filtrate, adjust the pH of the filtrate to 7-8 with concentrated hydrochl...
Embodiment 2
[0034] Under ice-bath conditions, add 75ml of 98% concentrated sulfuric acid to a 250ml three-necked flask with a thermometer, and then add 7.5ml of 65% concentrated nitric acid while stirring, and continue stirring for 10min. When the temperature of the reaction solution dropped to 5°C, 7.5 g of amantadine hydrochloride was added in portions, and after the addition was complete, the reaction was continued for 2 h in the ice bath, and then the ice bath was removed, and the reaction was continued for 2 h at room temperature. Transfer the system to a 500ml three-neck flask, add 120g of crushed ice while stirring, until the reaction solution turns clear dark green, put in an ice bath, add an appropriate amount of potassium hydroxide solid, adjust the pH of the solution to > 12, and keep The reaction was continued for 1 h under ice-bath conditions, and a large amount of white thick solid was produced. Suction filtration, collect the filtrate, adjust the pH of the filtrate to 7-8 w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com