Preparation method of high-purity vildagliptin
A high-purity, crude product technology, applied in the direction of organic chemistry, can solve the problems of low acetylation yield, difficulty in removing by-products, and small residual amount of 3-amino-1-adamantanol, so as to reduce the content and reduce the The effect of impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) Synthesis of vildagliptin crude product
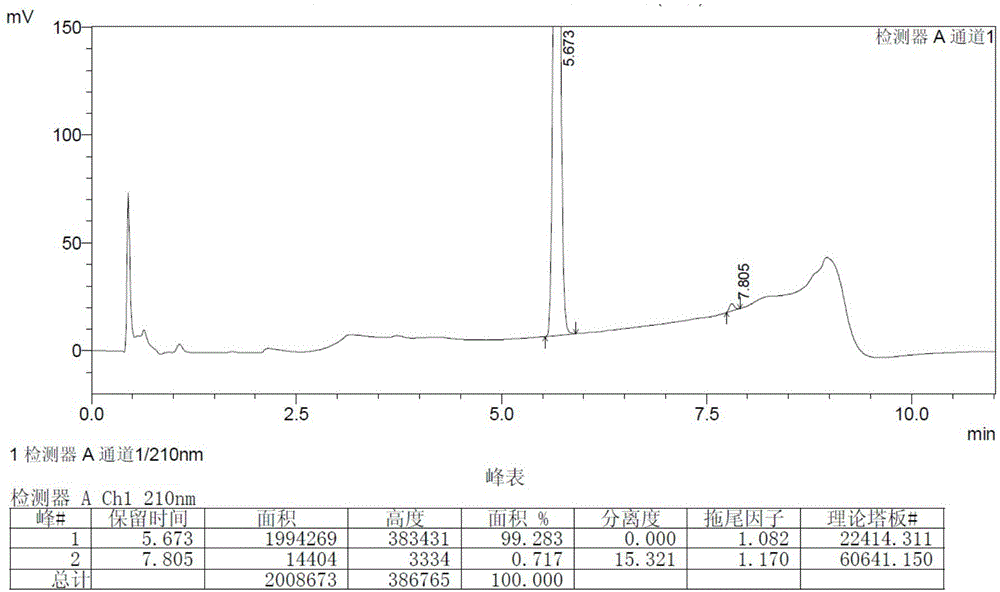
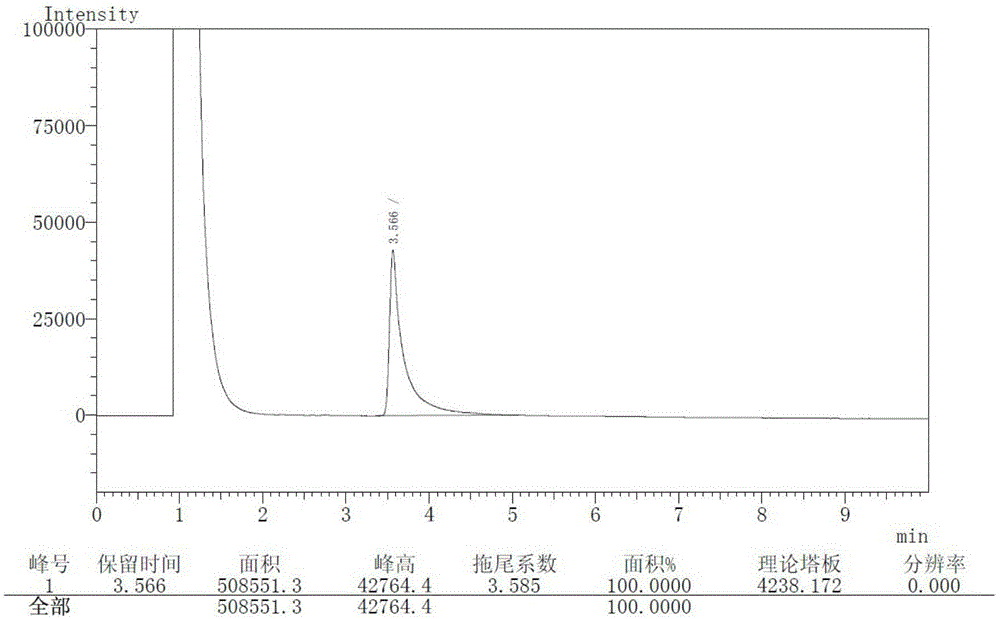
[0036] Add 500ml of tetrahydrofuran and 92g of 3-amino-1-adamantanol into a 1L three-necked flask, stir to dissolve the solid, then add 138g of potassium carbonate and 1.7g of potassium iodide, stir to cool down to 0°C to 5°C, add (2S)- 86.3 g of 1-(2-chloroacetyl)-2-pyrrolidinecarbonitrile. The temperature was controlled and the reaction was stirred for 10 hours, and the oil was obtained after post-treatment. Vildagliptin crude product 114.2g was obtained by crystallization of 2-butanone, with a yield of 75.3%. The HPLC and GC spectra of the crude product are respectively shown in the attached figure 1 And attached image 3 .
[0037] (2) Crude refined
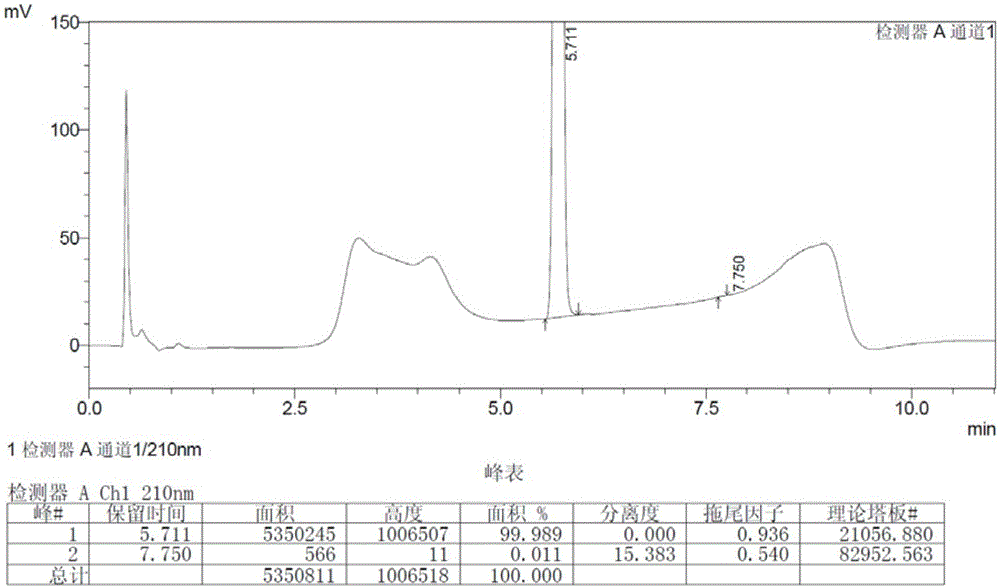
[0038] Add the crude product (100g) into 2-butanone (1.6L) and isopropanol (0.64L), heat to 60-70°C to dissolve the solid, filter, cool to 0-10°C to crystallize for 6 hours, and filter with suction. Dry under reduced pressure at 50°C to obtain 67.4g of refined product o...
Embodiment 2
[0040] (1) Synthesis of vildagliptin crude product
[0041] Add 1000ml of tetrahydrofuran and 192.3g of 3-amino-1-adamantanol into a 2L three-necked flask, stir to dissolve the solid, then add 276g of potassium carbonate and 3.4g of potassium iodide, stir to cool down to 0°C to 5°C, add (2S) - 172.6 g of 1-(2-chloroacetyl)-2-pyrrolidinecarbonitrile. The reaction was carried out under temperature control and stirring for 10 hours, and after treatment, an oily substance was obtained, and 218.8 g of crude vildagliptin was obtained by crystallization with isopropanol, with a yield of 72.1%.
[0042] (1) Crude refined
[0043] Add the crude product (200g) to 2-butanone (3.4L) and isopropanol (1.3L), heat to 60-70°C to dissolve the solid, filter, cool to 0-10°C to crystallize for 6 hours, and filter with suction. Drying under reduced pressure at 50°C yielded 138.4 g of white crystal refined product with a yield of 69.2%.
Embodiment 3
[0045] (1) Synthesis of vildagliptin crude product
[0046] Add 120ml of dichloromethane and 21g of 3-amino-1-adamantanol into a 500ml three-necked flask, stir to dissolve the solid, then add 33g of anhydrous potassium carbonate and 0.4g of potassium iodide, stir to cool down to 5°C-10°C, add 20.6 g of (2S)-1-(2-chloroacetyl)-2-pyrrolidinecarbonitrile. The reaction was carried out under temperature control and stirring for 12 hours, and post-treatment was carried out to obtain an oily product, which was crystallized with 2-butanone to obtain 25.5 g of crude vildagliptin, with a yield of 70.5%.
[0047] (2) Crude refined
[0048]Add the crude product (20g) to 2-butanone (300ml) and isopropanol (120ml), heat to 60-70°C to dissolve the solid, filter, cool to 0-10°C to crystallize for 6 hours, suction filter, 50°C After drying under reduced pressure, 13.7 g of white crystalline refined product was obtained, with a yield of 68.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com