Synthesizing method of vinorelbine tartrate
A technology of vinorelbine tartrate and a synthesis method, which is applied in the field of synthesis of vinblastine and vindoline to synthesize vinorelbine tartrate, can solve the problems of high price of synthetic raw materials, difficulty in obtaining, etc., and achieves cost reduction, cheap raw materials, and reduced production. cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
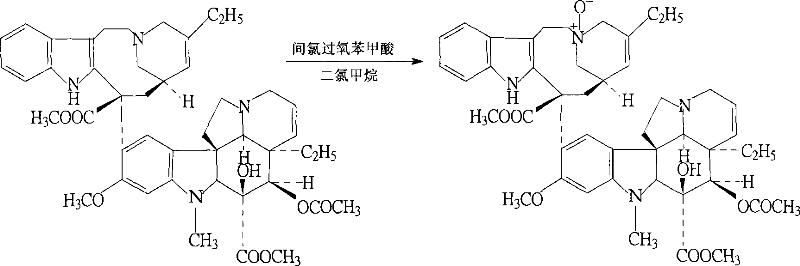
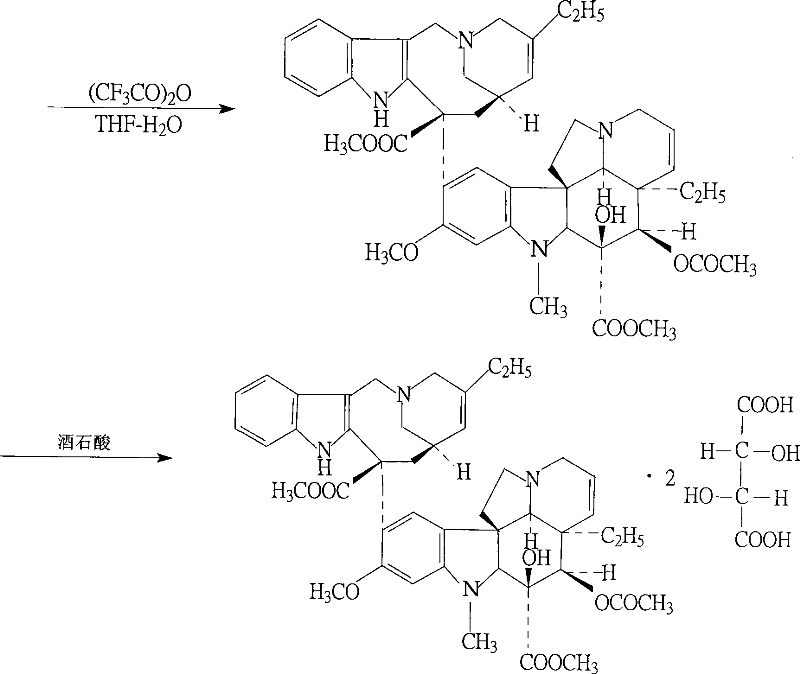
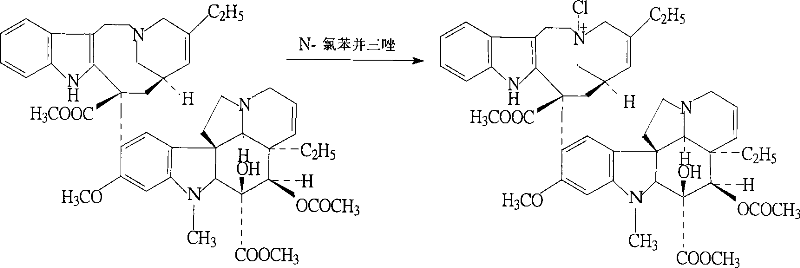
[0029] The present invention will be further described below in conjunction with specific embodiments.
[0030] (1) In a round bottom flask, add 34.8g of vinblastine tartrate (equivalent to 23.9g of vinblastine (M:336.43)) and 32.5g of vindoline, then add a small amount of dilute hydrochloric acid to dissolve, add 4.7L of trichloro Mix the iron oxide thoroughly and react for 13 hours under a sealed condition of 4°C. The reaction is terminated. A suitable amount of ammonia is added to the reaction solution to adjust the pH to 7, the reaction solution is centrifuged at high speed and the supernatant is discarded. The lower layer is soaked in chloroform several times. The chloroform phase is collected and evaporated to dryness to obtain the compound, which is the product of the first step reaction.
[0031] (2) Add 0.4 mol of hydrochloric acid to the product obtained after the chloroform phase is evaporated and dry, then add 5.7L of sodium borohydride to the dissolved material, react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com