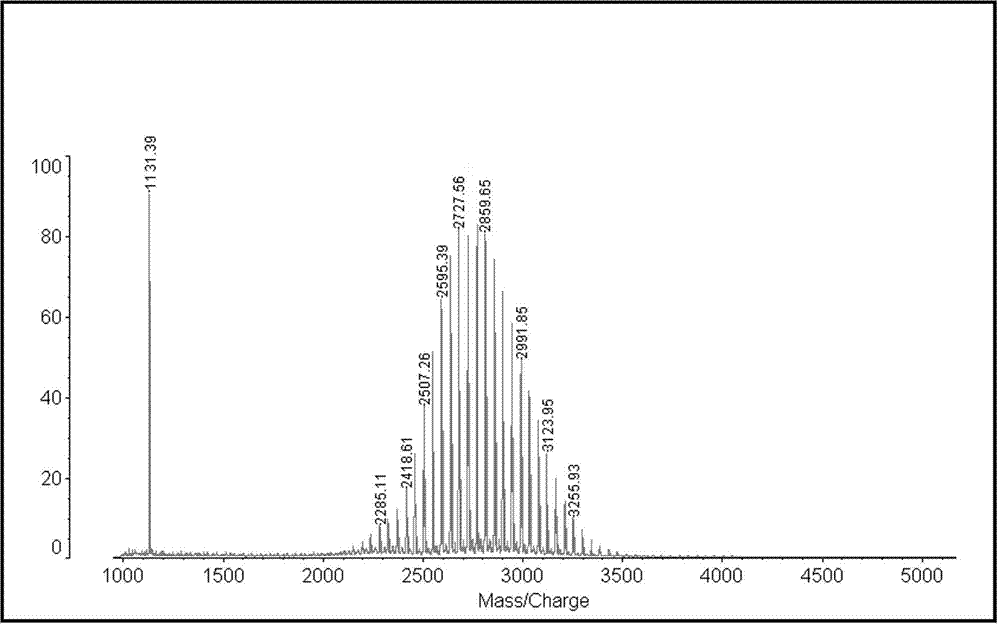
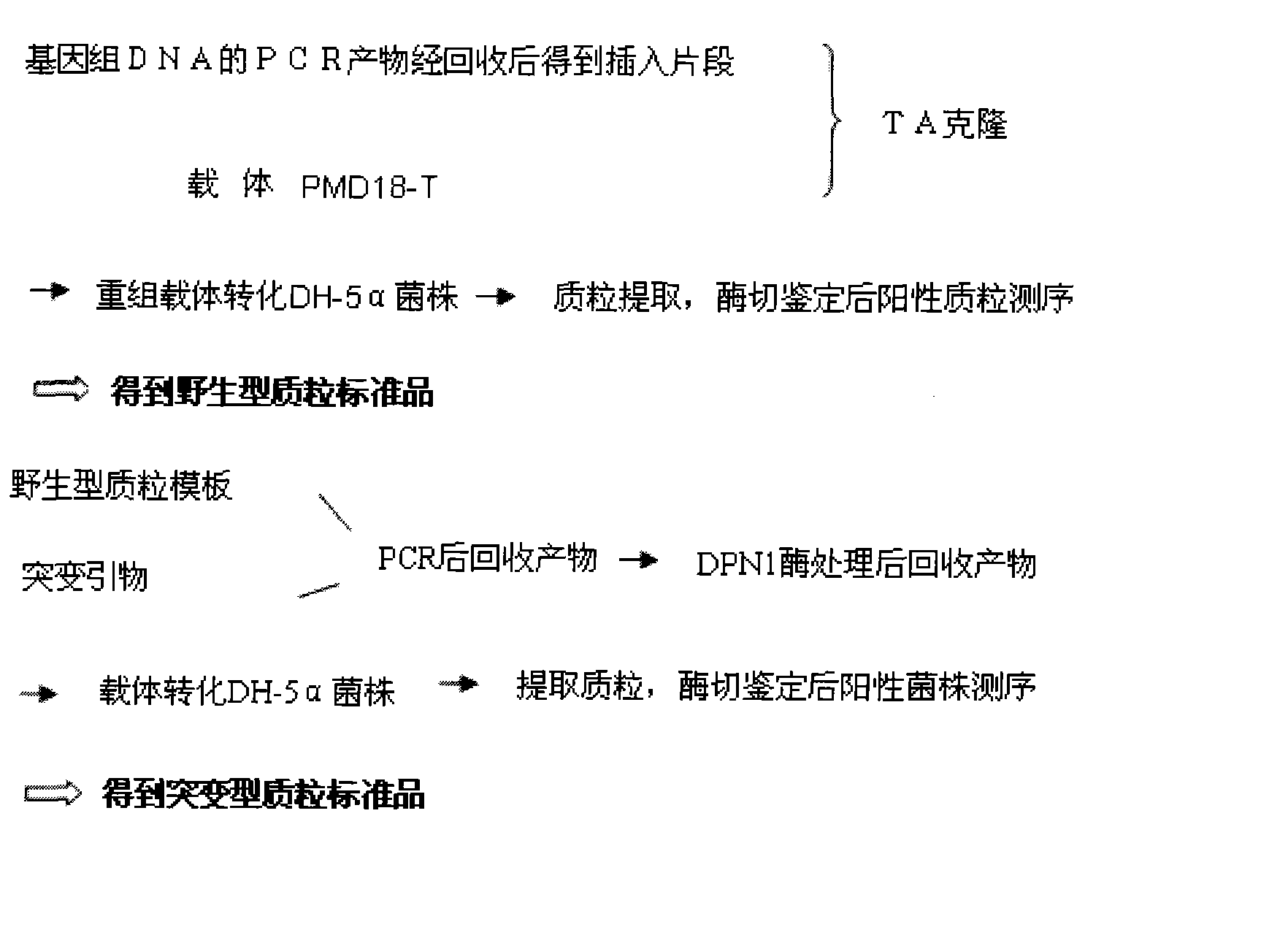
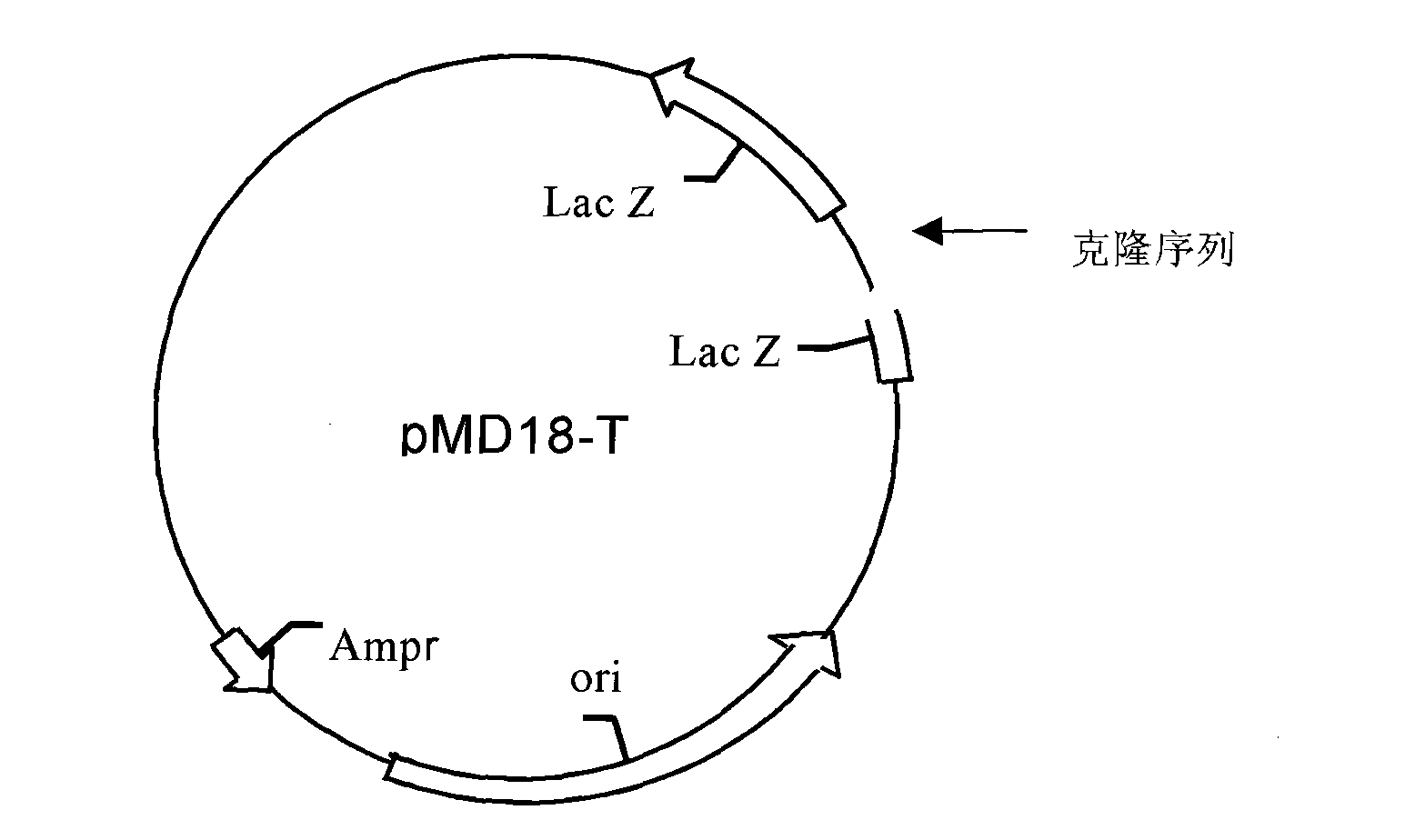
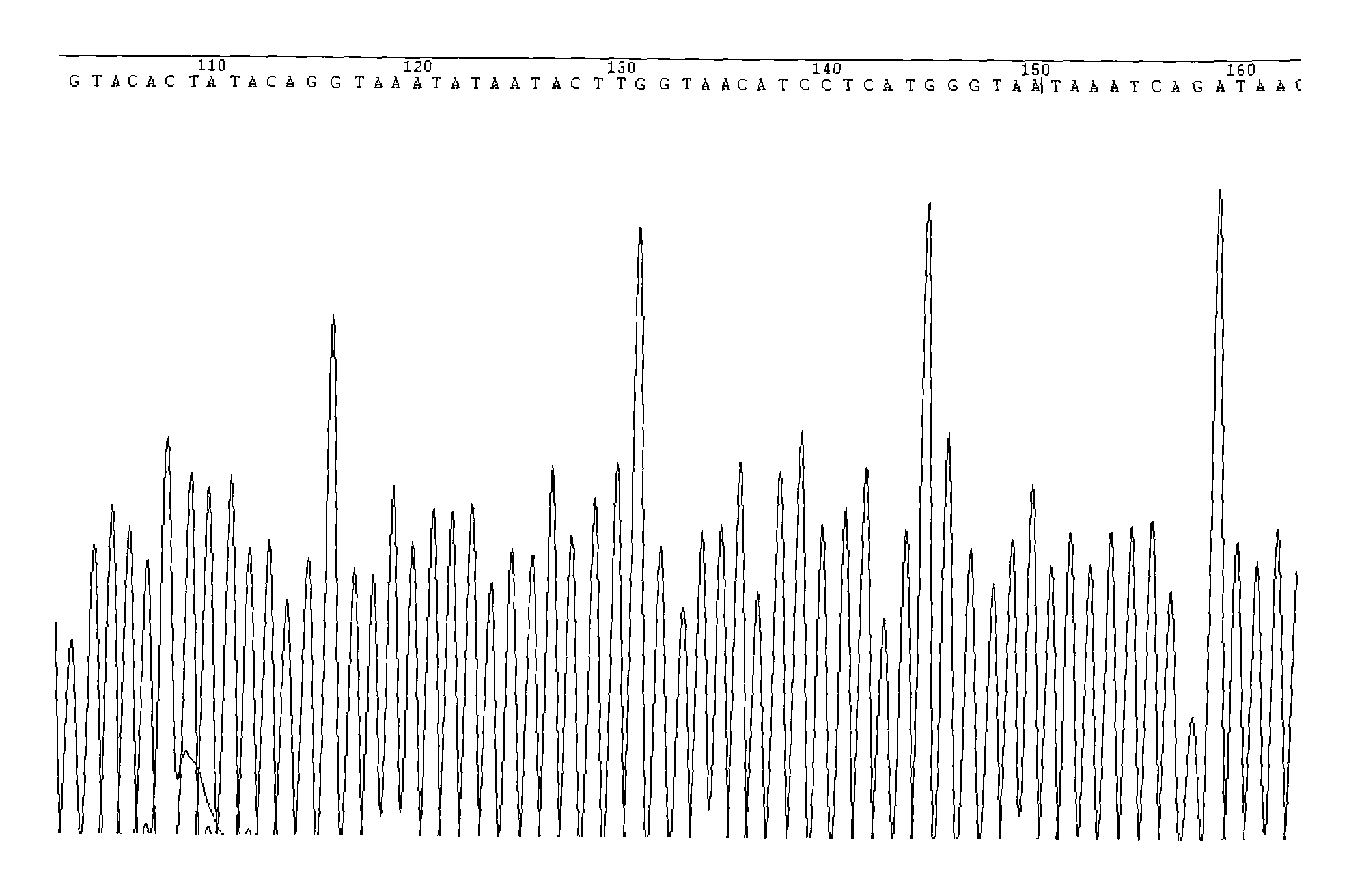
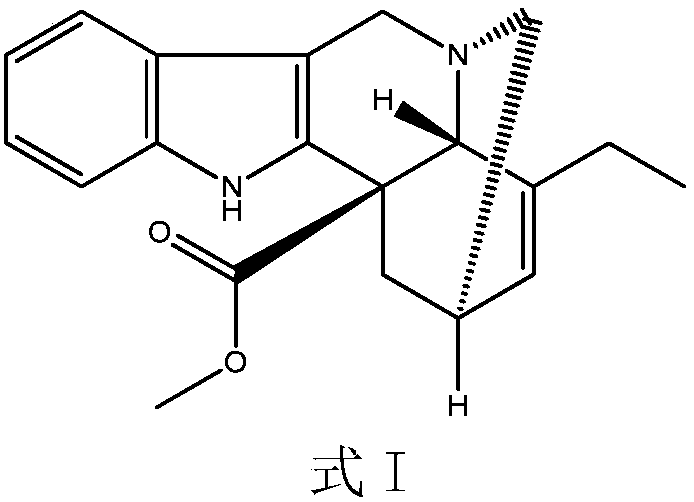
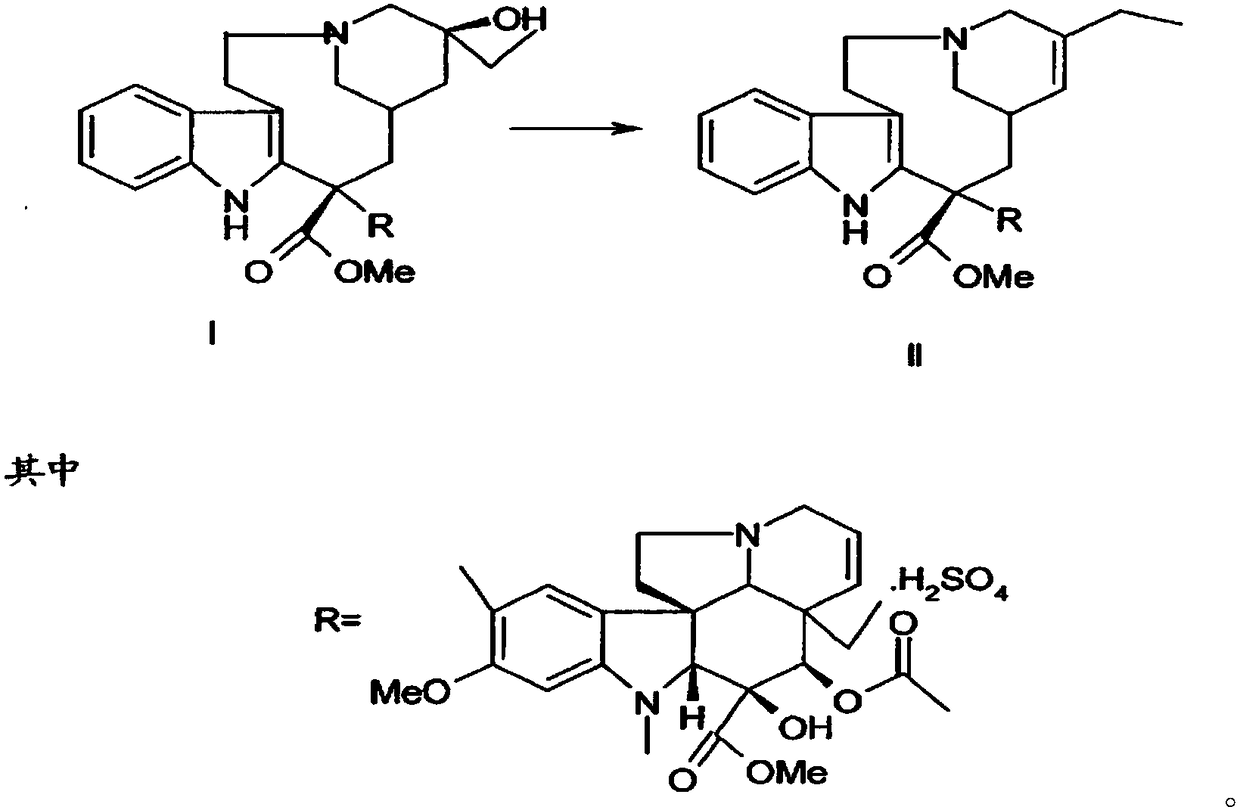
Patents
Literature
40 results about "Vindoline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
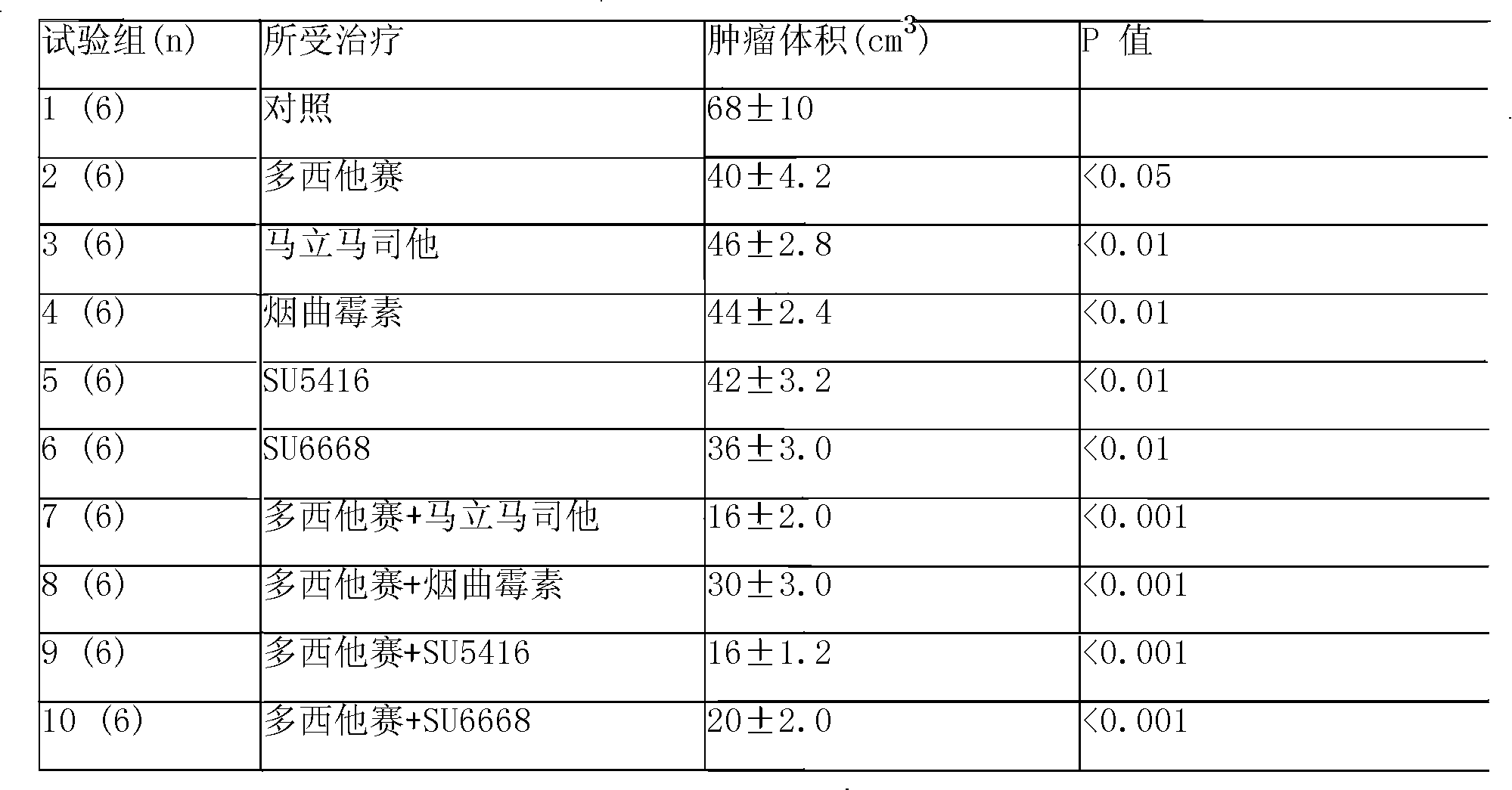
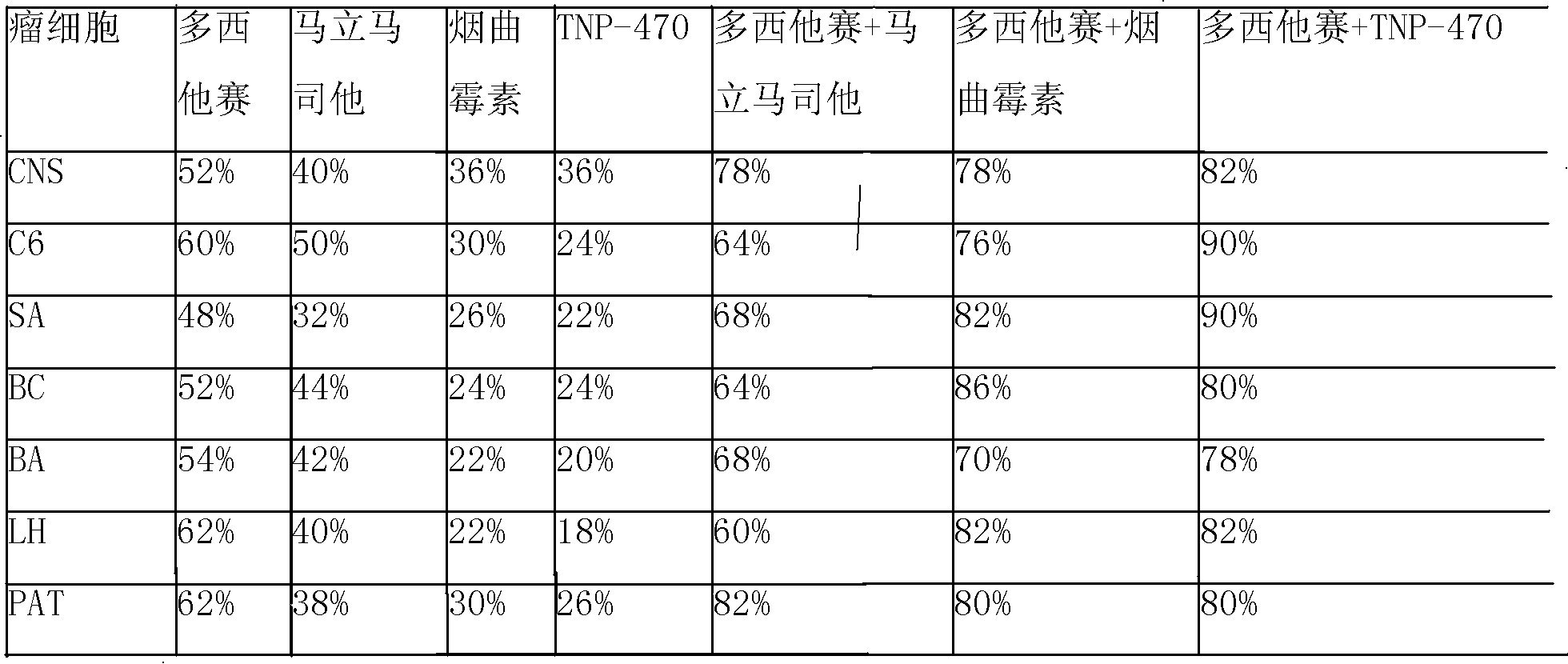
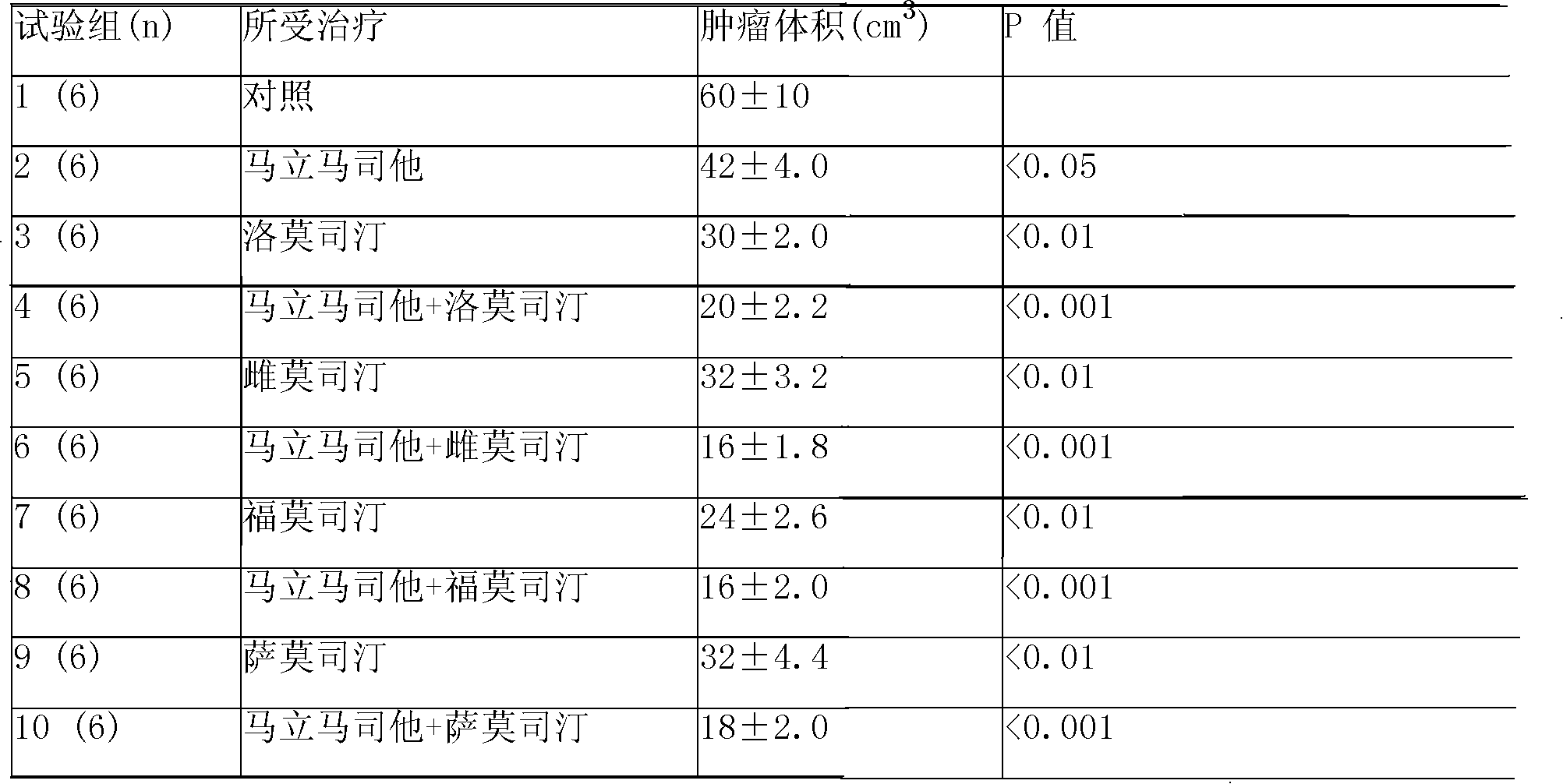
Vindoline is a chemical precursor to vinblastine. Vindoline is formed through biosynthesis from Tabersonine.
Folate-targeted diagnostics and treatment
InactiveUS20140140925A1Good energyShort half-lifePeptide/protein ingredientsRadioactive preparation carriersFolate targetingOncology
Methods of detecting and assessing functionally active folate receptors on tumors and treatment associated with those tumors are described. Also described are methods of selecting ovarian and lung cancer patients for therapy with a folate-vinca conjugate by identifying functionally active folate receptors on the tumors of the patient. Also described are methods and compositions for treating folate receptor expressing epithelial tumors with a folate-vinca conjugate in combination with doxorubicin such as pegylated liposomal doxorubicin in which the tumors include ovarian, endometrial or non-small cell lung cancer tumors, including platinum-resistant ovarian tumors and platinum sensitive ovarian tumors. Also described are methods of treating platinum-resistant ovarian cancer using a folate-targeted drug, in the absence or presence of selecting the patient by identifying functionally active folate receptors on the tumors of the patient.
Owner:ENDOCTYE INC
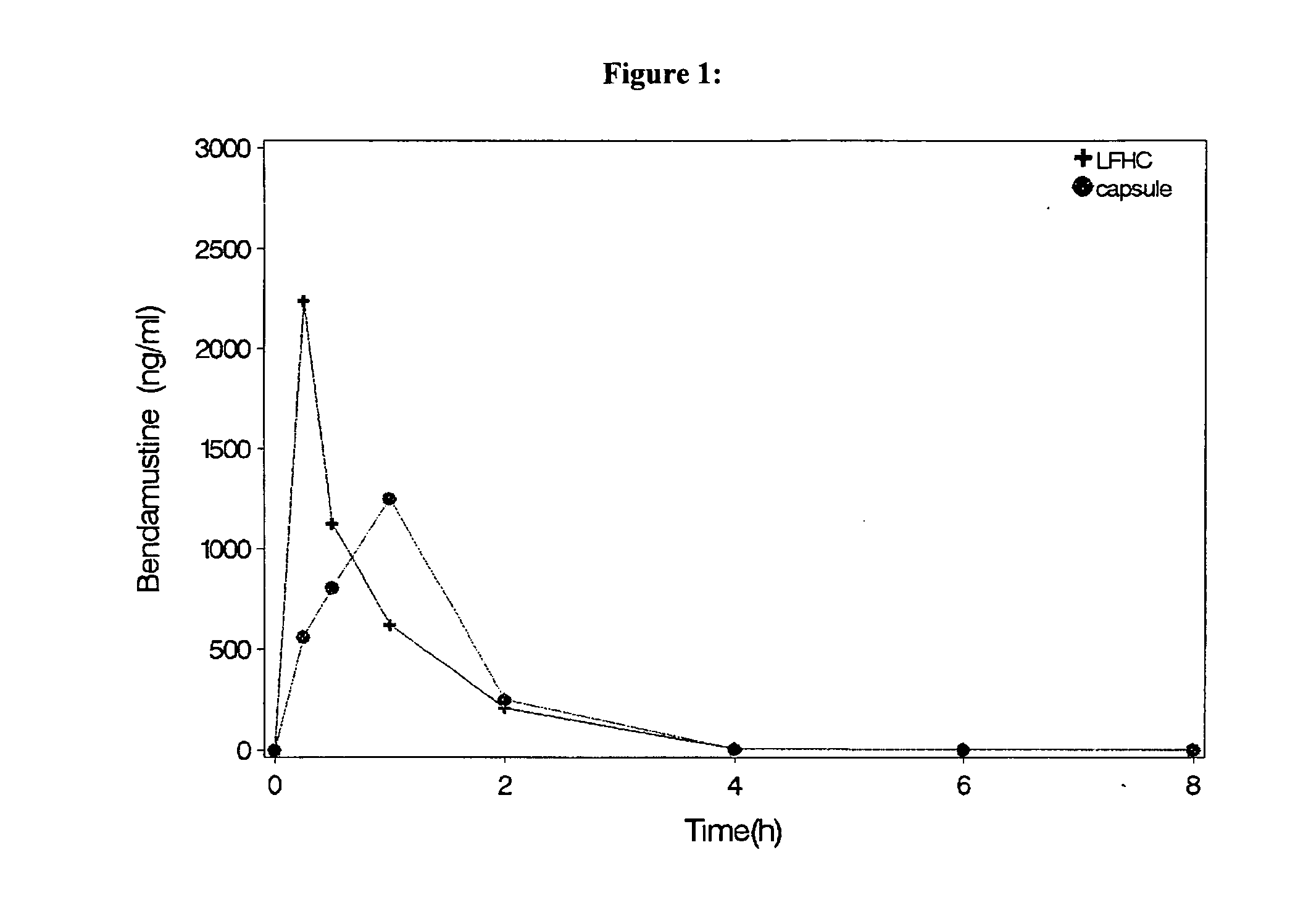
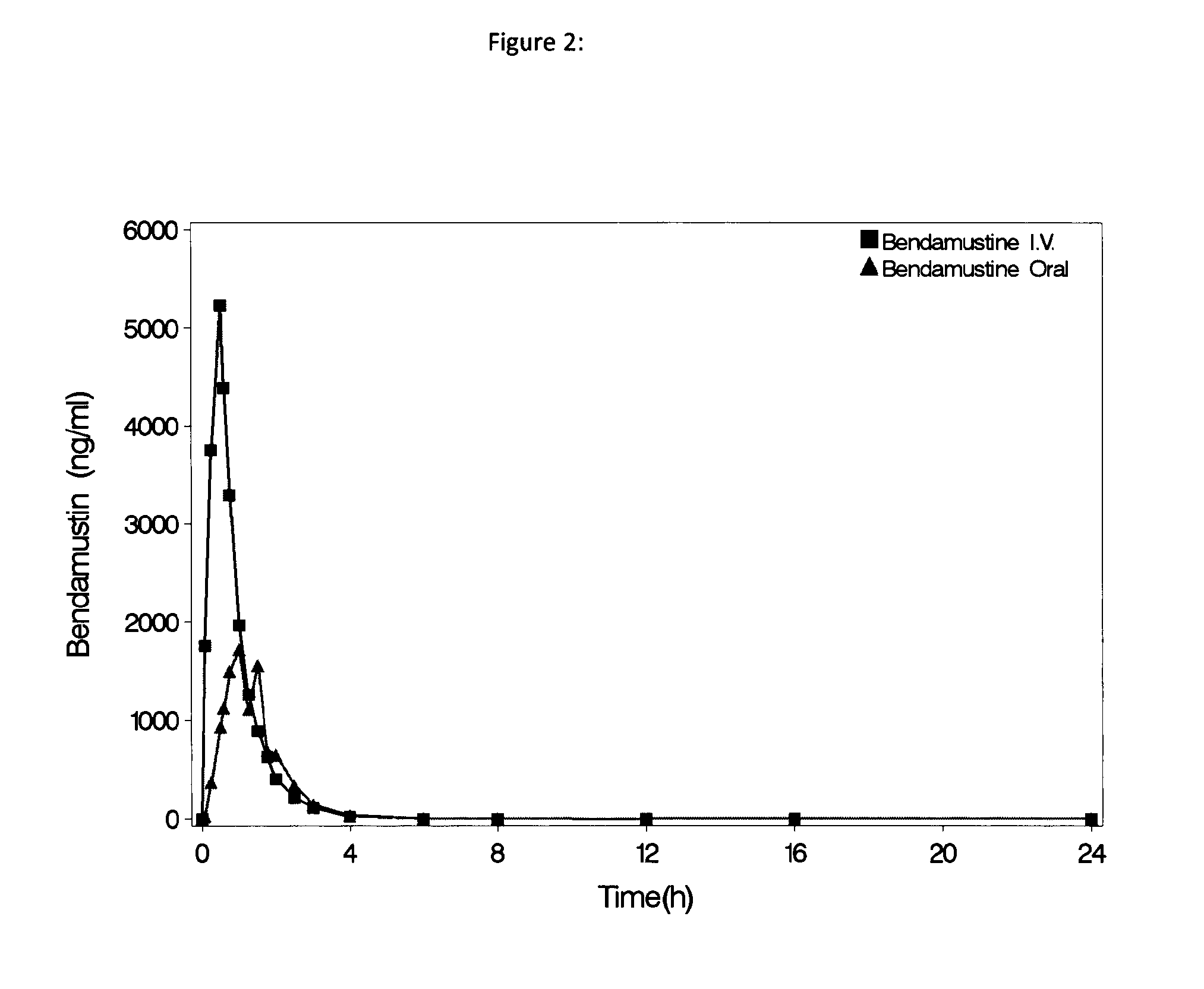
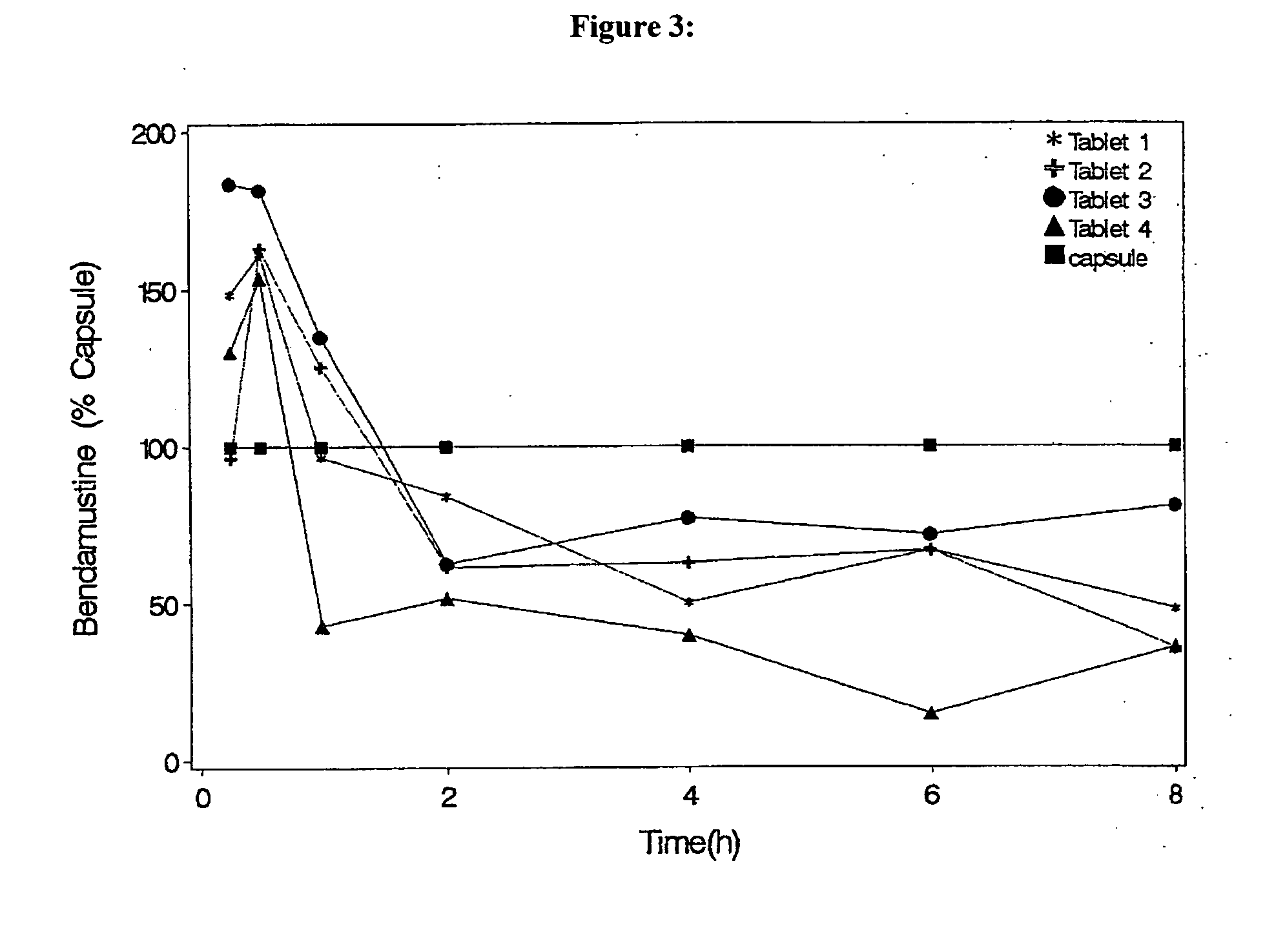
Oral Dosage Forms of Bendamustine and Therapeutic Use Thereof
InactiveUS20130209558A1Improve stabilitySuperior dissolution profileBiocideHeavy metal active ingredientsDiseaseOral treatment
In the present invention there is provided a pharmaceutical composition for oral administration which comprises bendamustine or a pharmaceutically acceptable, ester, salt or solvate thereof as an active ingredient, and a pharmaceutically acceptable excipient and which shows a dissolution of the bendamustine of at least 60% in 20 minutes, 70% in 40 minutes and 80% in 60 minutes, as measured with a paddle apparatus at 50 rpm according to the European Pharmacopoeia in 500 ml of a dissolution medium at a pH of 1.5, and wherein the pharmaceutically acceptable excipient is either a pharmaceutically acceptable non-ionic surfactant, selected from the group consisting of a polyethoxylated castor oil or derivative thereof and a block copolymer of ethylene oxide and propylene oxide or a pharmaceutically acceptable saccharide selected from the group consisting of one or more of a monosaccharide, a disaccharide, an oligosaccharide, a cyclic oligosaccharide, a polysaccharide and a saccharide alcohol, wherein the ratio by weight of the active ingredient to the saccharide excipient(s) is in the range of 1:1-5. The invention further relates to the above pharmaceutical composition for use for the oral treatment of a medical condition which is selected from chronic lymphocytic leukemia, acute lymphocytic leukaemia, chronic myelocytic leukaemia, acute myelocytic leukaemia, Hodgkin's disease, non-Hodgkin's lymphoma, multiple myeloma, breast cancer, ovarian cancer, small cell lung cancer and non-small cell lung cancer. The invention moreover relates to the above pharmaceutical composition for the above use wherein the dosage regimen comprises at least the administration of a dose of 100 to 600 mg / m2 / per person of bendamustine on day 1 and day 2, optionally a dose of 50 to 150 mg / m2 i.v. or orally of a corticosteroid on days 1 to 5, and optionally a suitable dose of a further active agent selected from the group consisting of an antibody specific for CD20, an anthracyclin derivative, a vinca alkaloid or a platin derivative; and the repetition of said dosage regimen 4 to 15 times after intervals of two to four weeks.
Owner:ASTELLAS DEUTLAND
Preparation method of vinorelbine
The invention belongs to the field of pharmaceutical synthesis, and provides a novel method used for synthesizing vinorelbine tartrate. According to the novel method, catharanthine tartrate and vindoline are taken as initial raw materials, vinorelbine crude products are obtained via a two-step one-pot procedure reaction instead of an original four-step reaction, synthesis steps are simplified, and yield is increased. The vinorelbine crude products are subjected to normal phase silica gel column purification and reverse phase silica gel column purification, so that purity of obtained vinorelbine purified products is increased substantially; and at the same time, increasing of vinorelbine purity possesses significant influences on salifying effects of vinorelbine with tartaric acid, so that after recrystallization, purity of the obtained vinorelbine tartrate is higher than 99.5%, and accords with requirements of United States Pharmacopeia; competitive advantage is more excellent; all of the operation processes are normal operation processes; and it is more beneficial for industrialized production.
Owner:NAT INST OF PHARMA R & D CO LTD
Combined treatment with docetaxel and an epidermal growth factor receptor kinase inhibitor using an intermittent dosing regimen
InactiveUS20070099856A1BiocideCarbohydrate active ingredientsAbnormal tissue growthEpidermal Growth Factor Receptor Kinase
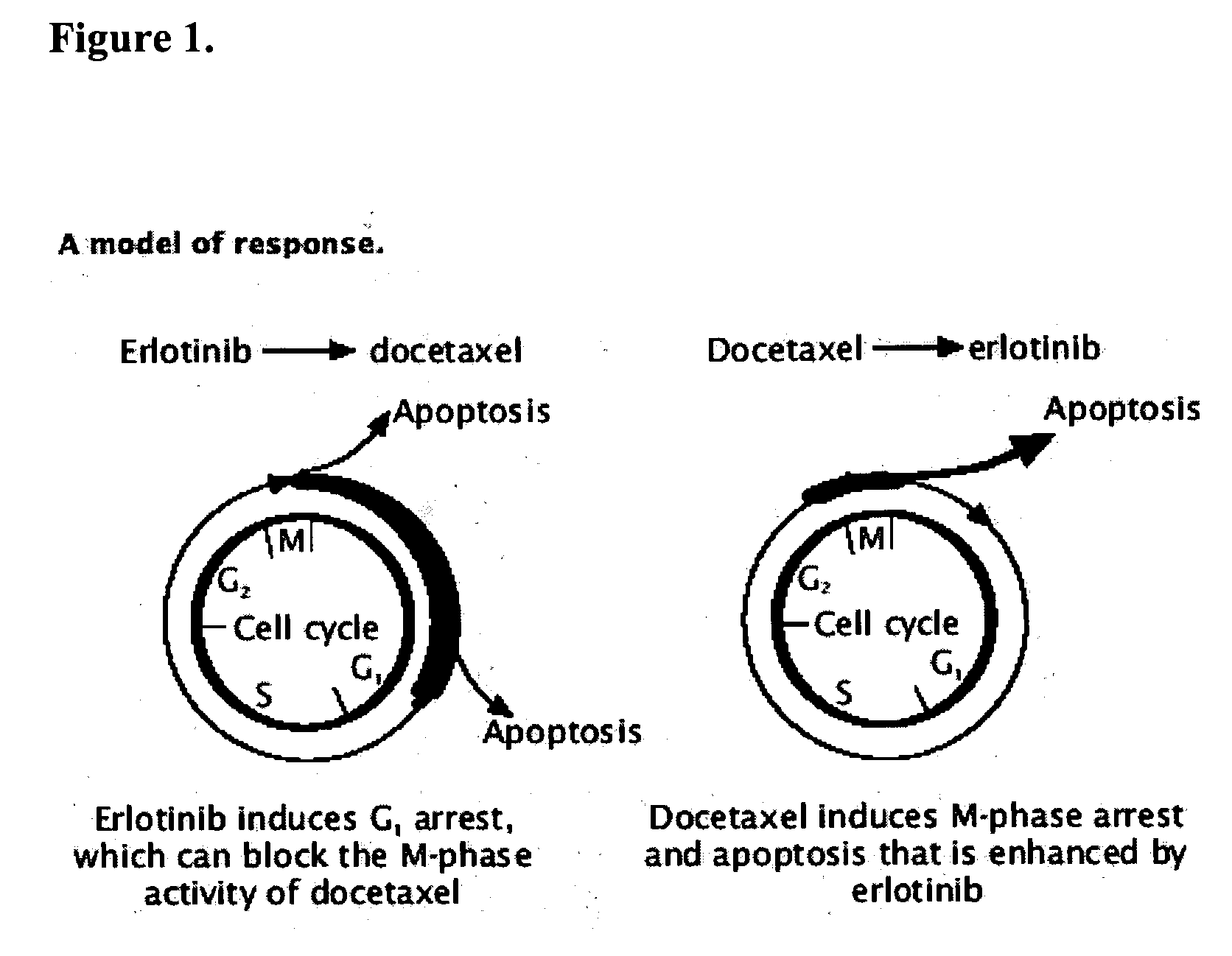
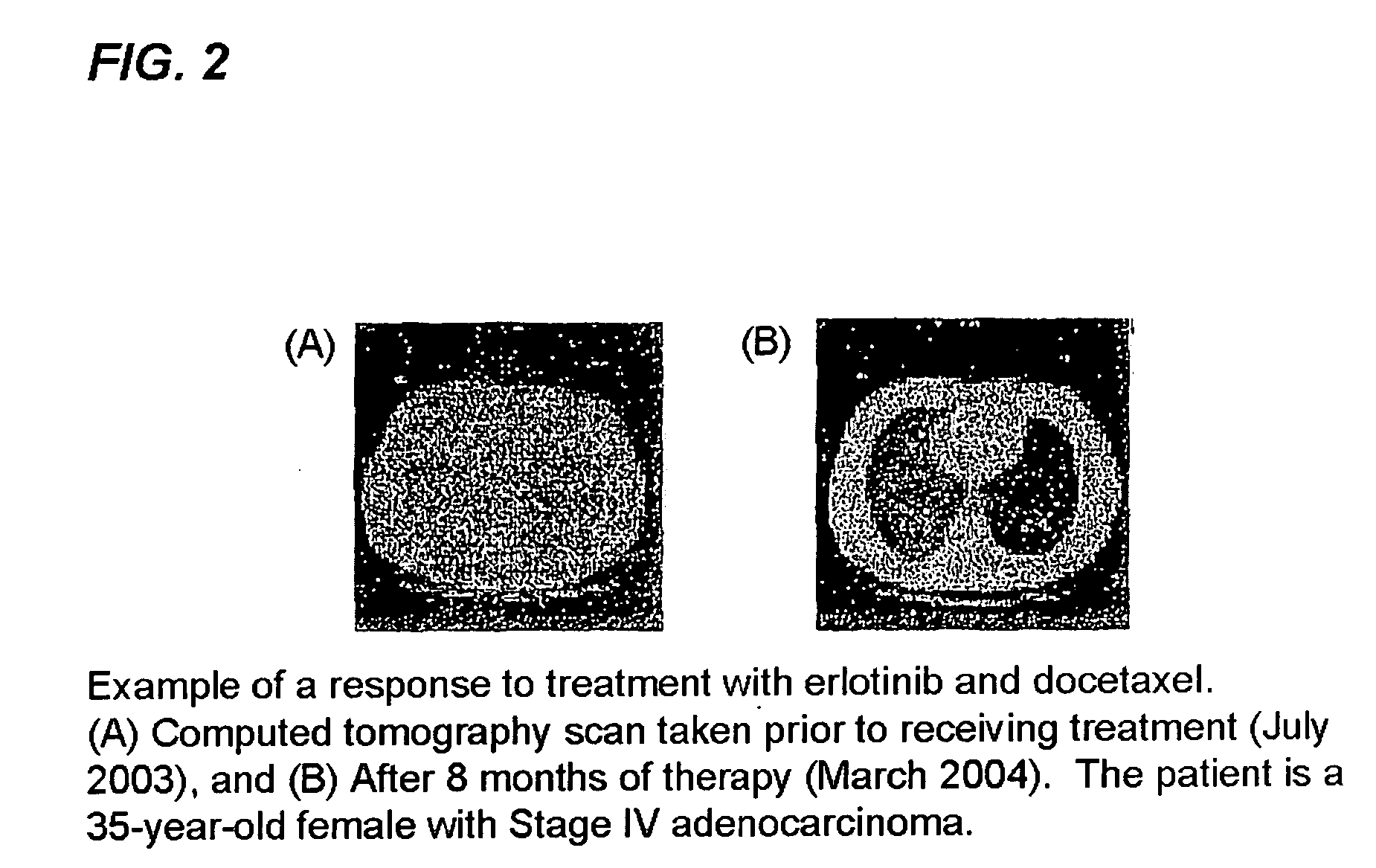
The present invention provides a method for treating tumors or tumor metastases in a patient, comprising administering to the patient a therapeutically effective amount of an EGFR kinase inhibitor and docetaxel combination, with or without additional agents or treatments, such as other anti-cancer drugs or radiation therapy, wherein the EGFR kinase inhibitor is administered intermittently after administration of a dose of docetaxel. Multiple cycles of this treatment can be administered until an effective result is obtained. Other anti-cancer agents that, like docetaxel, induce M-phase arrest, can be also used in the practice of this invention, e.g. vinblastine, paclitaxel. A preferred example of an EGFR kinase inhibitor that can be used in practicing this invention is the compound erlotinib HCl (also known as TARCEVA™).
Owner:RGT UNIV OF CALIFORNIA
Fluorouracil containing anti-cancer sustained-release injection
InactiveCN101234084AEasy to operateGood repeatabilityOrganic active ingredientsPharmaceutical delivery mechanismPolyethylene glycolSuspending Agents
The invention relates to anticancer sustained release injection which comprises sustained release microspheres and menstruum, wherein, the sustained release microspheres comprise anticancer active components and sustained release auxiliary material; the menstruum is special menstruum that contains suspending agent. The anticancer active components are fotemustine, nimustine, carmustine or combination of bendamustine and mitozolomide, docetaxel, etoposide, teniposide, vinblastine, anastrozole, tamoxifen, fluorouracil or mitomycin C; the sustained release auxiliary material is polylactic acid and polylactic acid copolymer, polyethylene glycol and polylactic acid copolymer of polyethylene glycol, terminal carboxyl group polylactic acid copolymer, EVAc, fatty acid and decanedioic acid copolymer, etc.; viscosity of the suspending agent is 100cp-3,000cp (at 25 DEG C-30 DEG C), and the suspending agent is selected from sodium carboxymethylcellulose, etc. The sustained release microspheres can also be made into sustained release implant; the injection or implant is injected or placed in or around tumor so as to reduce general reaction of the drug and selectively improve and keep local concentration for about 30-50 days. The anticancer sustained release injection can be used solely and can also promote anti-tumor effects of non-operative treatments, such as chemotherapy and / or radiotherapy, etc.
Owner:JINAN SHUAIHUA PHARMA TECH
Temperature controlled sustained-release injection containing anti-cancer medicine
InactiveCN101273965APharmaceutical delivery mechanismPharmaceutical non-active ingredientsTherapeutic effectVinorelbine
The invention relates to a temperature-controlled sustained-release injection containing an anti-cancer drug, which consists of the anti-cancer drug and an amphiphilic block copolymer hydrogel and has the temperature-sensitive gelatinization feature, the temperature-controlled sustained-release injection is flowable liquid in the environment that is lower than the body temperature and can be automatically converted to the water-insoluble gel that can not flow and be biodegradable for absorption in an endotherm, thus allowing the drug to have the local sustained release in a tumor and maintain the effective drug concentration for a plurality of weeks to a plurality of months; the temperature-controlled sustained-release injection can be injected in the tumor or the tumor periphery or be arranged in the postoperative tumor cavity, thus significantly reducing the systemic reaction of the drug, strengthening the treatment effects of chemotherapy, radiotherapy and other non-surgical therapies, and being used for the treatment of the tumors in different stages. The anti-cancer drug can be vincristine, vinorelbine, navelbine, vindesine, vinleurosine, vinrosidine, cephalotaxine, bleomycin, daunomycin, aclarubicin, epirubicin, idarubicin, pirarubicin, valrubicin, mitomycin C, actinomycin D, losoxantrone, mitoxantrone, mitozolomide, temozolomide and so on.
Owner:SHANDONG LANJIN PHARMA +1
Polymer nanoparticles for loading alkaline antitumor drugs
InactiveCN102697735ADoes not change local pHGood curative effectPowder deliveryPharmaceutical non-active ingredientsDocetaxel-PNPSide effect
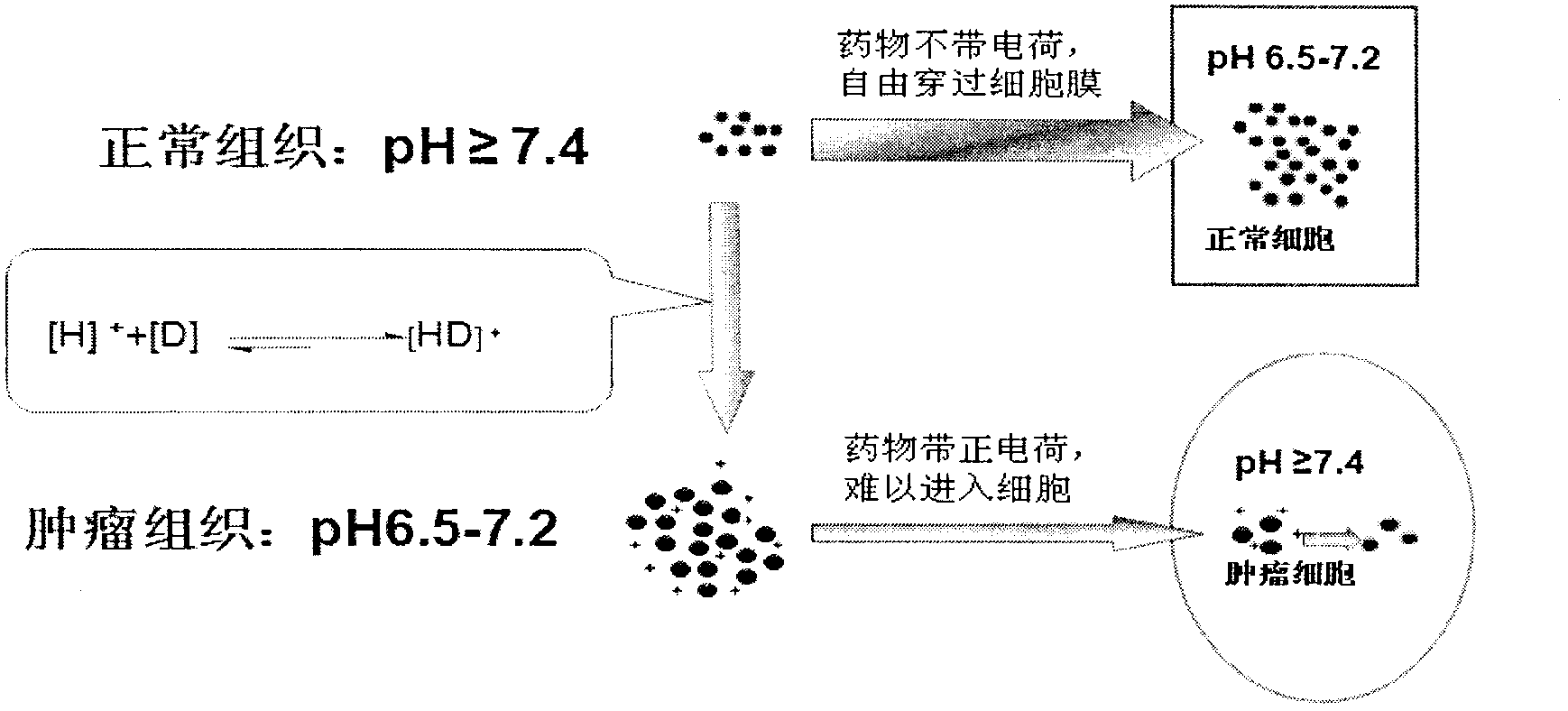
The invention relates to polymer nano-particles for loading alkaline antitumor drugs. A polymer material forming the nanoparticles has biological compatibility and biodegradability, and the polymer material loads the alkaline antitumor drugs; and the polymer material comprises liposome, polylactic acid, polylactic acid-glycolic acid copolymer, polycaprolactone, chitosan, albumin and cyclodextrin; and the alkaline antitumor drug is a micromolecule antitumor drug with pKa being more than 7.4 and comprises adriamycin, pharmorubicin, taxol, docetaxel, vinblastine, vincristine, topotecan and alkaloid Chinese medicine monomers. The polymer nano-particles solve the defects that the pH dependence and physiological drug fastness cannot be reversed. The polymer nano-particles are free from introducing additional drugs, so that the influence on the internal environment of an organism is small, the pH dependence physiological drug fastness is reversed through the polymer nano-particles, not only is the effect realized, but also since no influence is caused for the internal environment of the organism, the polymer nano-particles are safe to use, little in side effect, and wide in application range.
Owner:THE AFFILIATED DRUM TOWER HOSPITAL MEDICAL SCHOOL OF NANJING UNIV
Vincristine and method for synthesizing vincristine sulfate
The invention discloses vincristine and a method for synthesizing vincristine sulfate. The method for synthesizing vincristine comprises the following steps: 1) preparing vinblastine; and 2) synthesizing, purifying and refining vincristine, namely carrying out reaction on the synthesized vincristine and ethanol sulfate on the basis of the method for synthesizing vincristine, so as to obtain a crude product of the vincristine sulfate, and refining the crude product of the vincristine sulfate to obtain the vincristine sulfate. The vincristine is synthesized by adopting a one-step synthesis method when the vincristine is synthesized, the synthetic route of the vincristine is simplified, the method is simple in synthetic route, and low in production cost, the purity of the synthesized vincristine can be up to over 96% after the synthesized vincristine is refined, and the vincristine sulfate is synthesized on the basis of the method for synthesizing the vincristine. The process for synthesizing the vincristine is simple, so that the synthetic route of the vincristine sulfate is greatly simplified, the method is simple in synthetic route, and low in production cost, and the purity of the refined vincristine sulfate can be up to over 96%.
Owner:HUBEI HONCH PHARMA
Multifunctional targeting vinorebine lipidosome and preparation method thereof
ActiveCN104224718AImprove distributionResearch to aid in non-invasive treatmentsOrganic active ingredientsMacromolecular non-active ingredientsSolubilityLipid formation
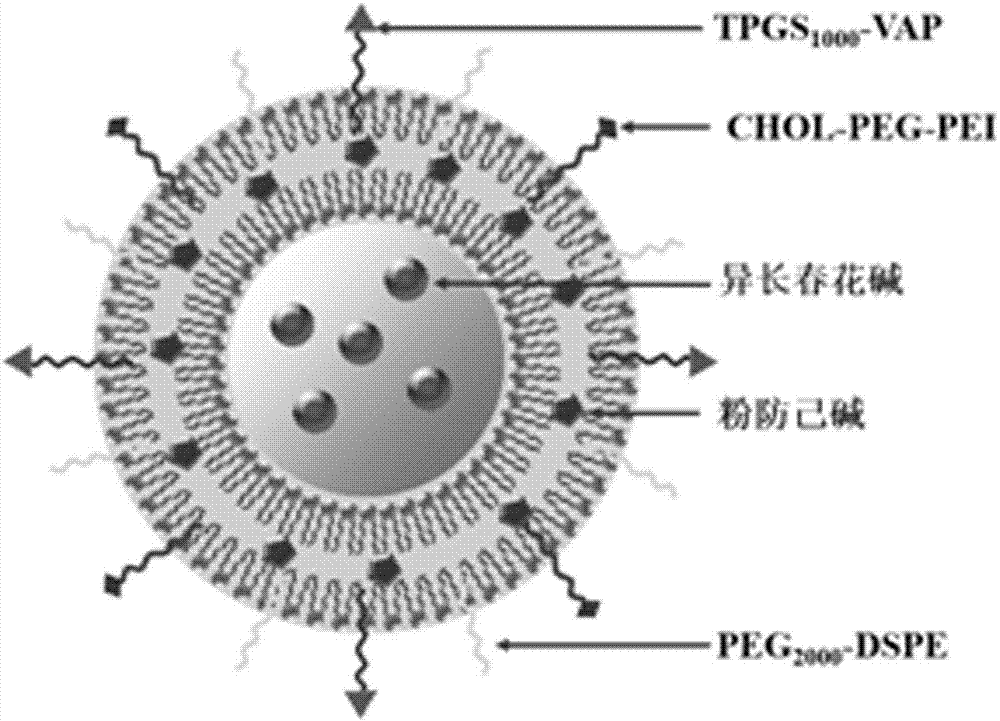
The invention discloses a multifunctional targeting vinorebine lipidosome which is composed of vinorebine, lipid materials and functional targeting ligand; the lipidosome is a bi-molecule structure in which a water-solubility cavity is arranged; vinorebine is encapsulated in the water-solubility cavity; the lipid materials are composed of lecithin, cholesterol, polyethylene glycol-DSPE; the functional targeting ligand is composed of modifications on the lipidosome surface and targeting materials in the bi-molecule layer of the lipidosome; the mentioned targeting materials are tetrandrine encapsulated in the bi-molecule layer of the lipidosome; the mentioned modifications are vapreotide-polyethylene glycol 1000 vitamin E succinate and cholesterol-polyethylene glycol-polyethyleneimine. The invention further discloses a preparation method of the multifunctional targeting vinorebine lipidosome, through the method, drugs can be targeted into brain glioma positions after being crossed over blood brain barriers, thereby increasing drug distribution in brain tumor positions and improving chemotherapeutic efficacy.
Owner:大连博格林生物科技有限公司
Method for preparing vindoline and Catharanthine
InactiveCN1660845AReduce processing costsSuitable for industrial productionOrganic chemistrySal ammoniacAlcohol
A process for preparing vindoline and vinblastine, which can be used to prepare the antineoplastic medicines, from Chinese ivy flower includes such steps as immersing in sulfuric acid solution, regulating pH=7-8, extracting in chloroform, vacuum concentrating, dissolving in absolute alcohol, adding the solution of sulfuric acid in alcohol antil pH=3.8-4.1, laying aside for educing out crystals, filtering to obtain vinblastine sulfate, regulating pH of fitlrate to 7-8, extracting in chlorofmr, vacuum concentrating, absorbing by macroreticular resin, eluting with mixture of polar solvent and water, and vacuum concentrating.
Owner:上海安体康生植物化学有限公司
Method for separating vindoline , catharanthine, vinblastine and vincristine from Vinca rosea
The invention relates to a method for separating catharanthine, eurosine and vincristin, which comprises: expelling the foreign matter of Catharanthus roseus, homogenate training the mushrooms, expelling the training liquid, hollowing the turbid liquor, ejecting the liquid, filtering, the extracting liquid negative pressure forming film condensation, double-water phase extracting, extracting liquid condensation, Catharanthus roseus total alkaloid, continues middle compression leg colour printing gradient cleaning to obtain the catharanthine, eurosine and vincristin. The eurosine uses negative pressure mold method to obtain the eurosine with high purity.
Owner:NORTHEAST FORESTRY UNIVERSITY +1
Kit for quantitatively detecting breast cancer resistance protein (BCRP) mutation
InactiveCN102134567AAccurate determination of contentEasy to operateMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceDaunorubicin
The invention relates to a method and a kit for detecting breast cancer resistance protein (BCRP) gene mutation related to treatment effect of molecular targeted anti-cancer medicines, in particular to a fluorescence quantitative polymerase chain reaction (PCR) detection method and a kit for detecting mutation in a BCRP genic mutation hotspot region, and application thereof. By the invention, the mutation of a specific BCRP gene locus is detected, the treatment effects of anti-tumor medicines such as mitoxantrone MX, adriamycin, daunorubicin, etoposide, topotecan, Irinotecan, CPT-11, cis-platinum, taxol, vinblastine and the like are predicted, and guidance is provided for clinical individualized medication schemes for patients suffering from tumors.
Owner:BEIJING ACCB BIOTECH
Method for producing vindoline
InactiveCN101333511AEasy to synthesizeIncrease contentFermentationPlant cellsBiotechnologyAcetic anhydride
The invention discloses a method for producing vindoline. The method comprises: inoculating a periwinkle polyploid cell strain in a synthetic medium, and obtaining a cell rich in vindoline by cultivating the strain for 3 to 7 days at the temperature between 20 and 28DEG C; and the synthetic medium is a liquid culture medium obtained by adding 0 to 4mg / L of plant cell auxin, 0 to 4mg / L of plant cytokinin, 30 to 60g / L of sucrose, 10 to 40mu g / L of acetyl coenzyme A, 0.5 to 1.5mu mol / L of Benzotriazole methl, 5 to 30mu mol / L of tranylcypromine, 10 to 40mu l / L of acetic anhydride and 10 to 40mg / L of dithiothreitol in a minimal medium. The method using periwinkle cells to cultivate and produce vindoline can stably realize the industrialized production of vindoline, and the production cost is rather low, the culture period is short, and the production is insusceptible to the natural environment and weather, so the year-round production can be realized.
Owner:TSINGHUA UNIV
Docetaxel-containing anti-cancer sustained-release injection
InactiveCN101234085AEasy to operateGood repeatabilityOrganic active ingredientsPharmaceutical delivery mechanismPolyethylene glycolSuspending Agents
The invention relates to anticancer sustained release injection which comprises sustained release microspheres and menstruum, wherein, the sustained release microspheres comprise anticancer active components and sustained release auxiliary material; the menstruum is special menstruum that contains suspending agent. The anticancer active components are fotemustine, nimustine, carmustine or combination of bendamustine and mitozolomide, docetaxel, etoposide, teniposide, vinblastine, anastrozole, tamoxifen, fluorouracil or mitomycin C; the sustained release auxiliary material is polylactic acid and polylactic acid copolymer, polyethylene glycol and polylactic acid copolymer of polyethylene glycol, terminal carboxyl group polylactic acid copolymer, EVAc, fatty acid and decanedioic acid copolymer, etc.; viscosity of the suspending agent is 100cp-3,000cp (at 25 DEG C-30 DEG C), and the suspending agent is selected from sodium carboxymethylcellulose, etc. The sustained release microspheres can also be made into sustained release implant; the injection or implant is injected or placed in or around tumor so as to reduce general reaction of the drug and selectively improve and keep local concentration for about 30-50 days. The anticancer sustained release injection can be used solely and can also promote anti-tumor effects of non-operative treatments, such as chemotherapy and / or radiotherapy, etc.
Owner:JINAN SHUAIHUA PHARMA TECH
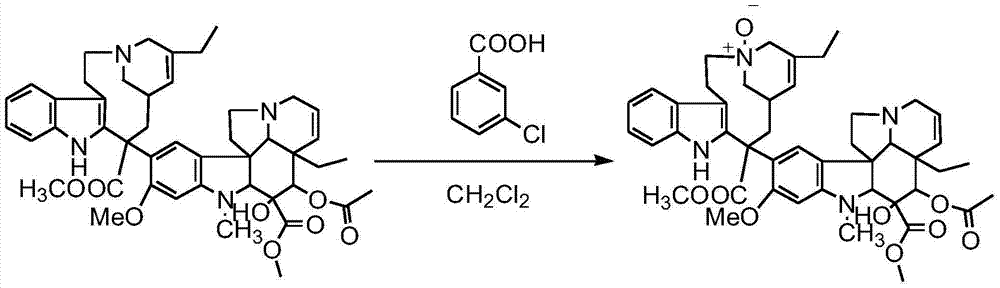
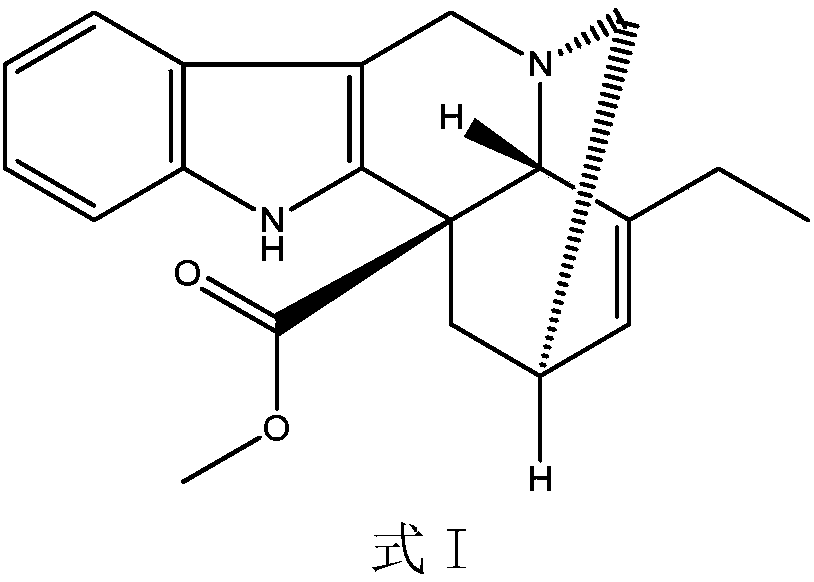
Preparation method of vinblastine derivative
ActiveCN106749342ASimple and fast operationSuitable for industrial productionOrganic chemistrySulfatePeroxide
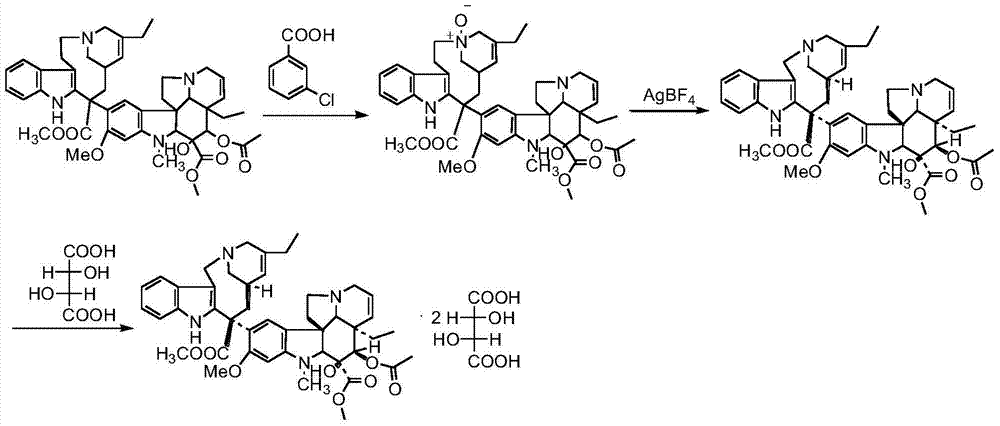
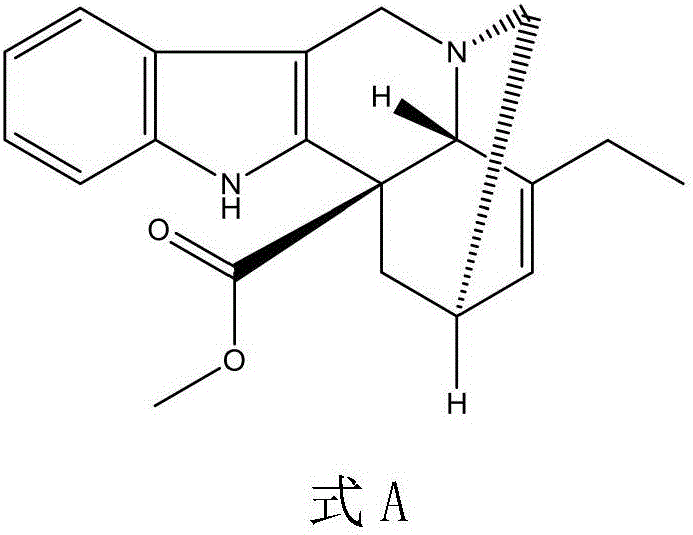
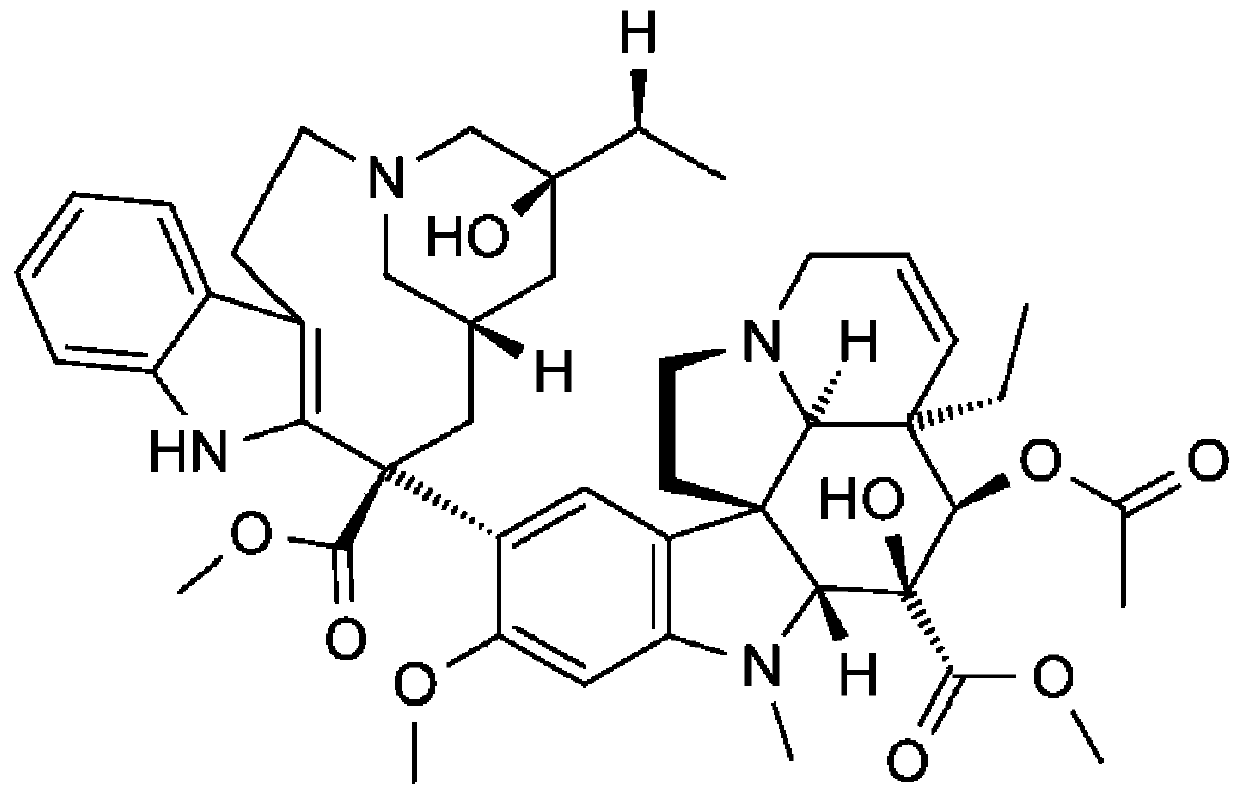
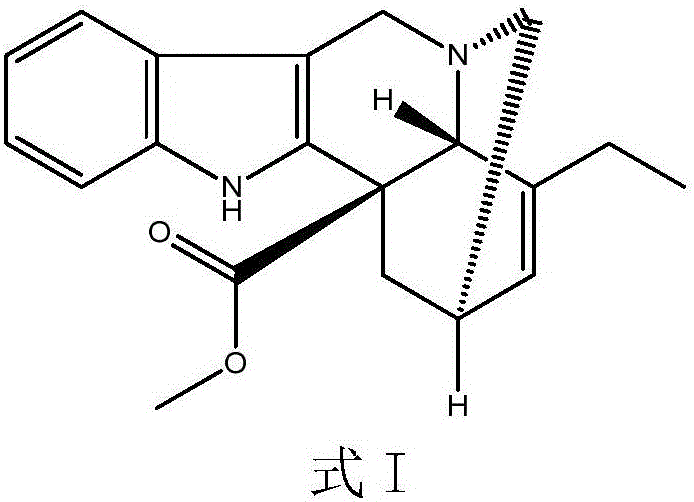
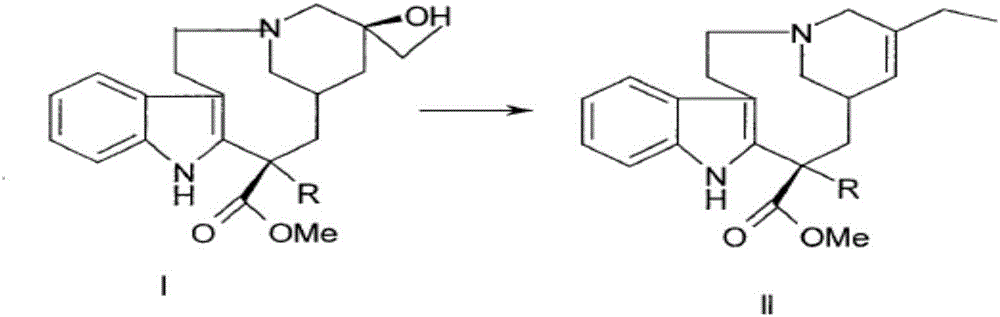
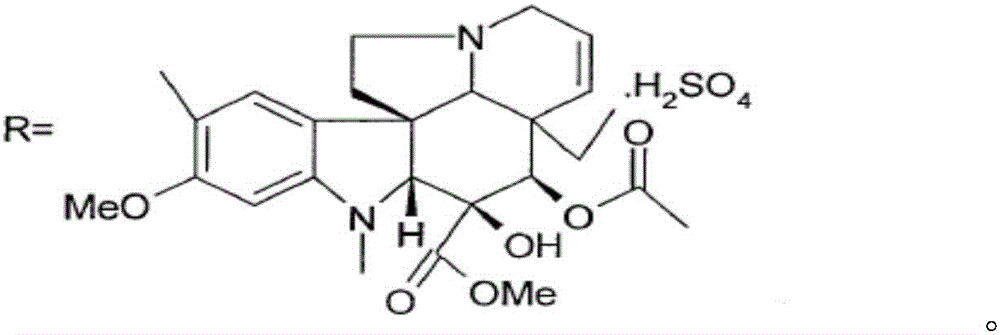
The invention relates to a preparation method of a vinblastine derivative. The preparation method comprises the following steps of under the effect of ferric sulfate and peroxide formic acid, a compound shown as the formula A is coupled with vindoline; then, a reducing agent is added for performing reduction reaction to obtain the navelbine through preparation. The preparation method has the advantages that the yield is high; the purity of the obtained product is very high.
Owner:河南绿园药业有限公司
Anti-cancer slow release injection comprising plant alkaloid
InactiveCN1923281APharmaceutical delivery mechanismAntineoplastic agentsSuspending AgentsVinorelbine
Disclosed is an anticancer slow release injection containing plant alkaloids, wherein the constituents include anti-cancer drugs, slow release auxiliary materials, suspending agent and / or dissolvent. The anti-cancer drugs include leurocristine, Vinblastine, procarbazine, leurosidine or vinorelbine, the slow release auxiliary materials can be selected from polylactic acid, glycolic acid and glycolic acid copolymer, ethylene-vinylacetate copolymer or their combination. The suspending agent is selected from sodium carboxymethylcellulose and mannitol. The dissolvent is selected from distilled water, water for injection, physiological lotion, absolute ethyl alcohol, microcosmic salt or carbonates cushioning liquid. The anticancer slow release injection can be administered through subcutaneous, intracavity, intra-tumor, tumor-surrounding, intra- lymph gland or bone marrow channels, the whole body toxicity reaction of the anti-cancer medicament can be lowered, the tumor local medicinal concentration can also be selectively increased and maintained, and the treatment effect of the non-operative treatment methods such as chemotherapy, medicament and radiation can also be improved.
Owner:孔庆忠
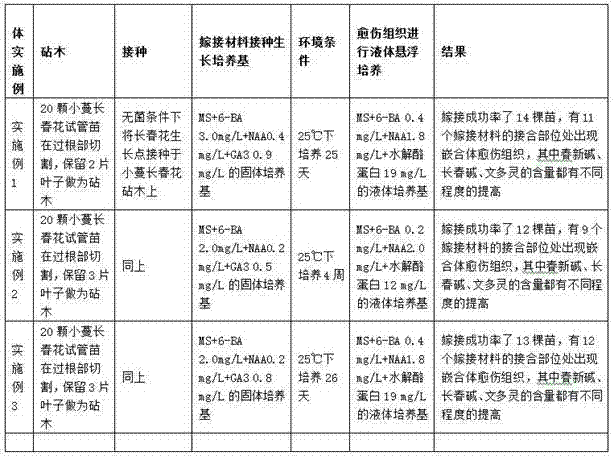
Method for building cell culture system for chimera obtained through grafting vinca rosea to vinca minor
The invention discloses a method for building a cell culture system for a chimera obtained through grafting vinca rosea to vinca minor. The method comprises the steps of cutting roots of the vinca minor, then, reserving 2 to 3 leaves for each vinca minor plant so as to prepare rootstock, inoculating growth points of the vinca rosea to the vinca minor rootstock under aseptic conditions, then, enabling each place of combination of a graft and the corresponding rootstock to emerge a callus, carrying out fluid suspension culture on the calli, and carrying out screening so as to obtain an excellent cell line. According to the method, after the growth points of the vinca rosea is grafted to the vinca minor, the calli are induced by using the obtained chimera as a material, and finally, an in-vitro cell suspension culture system for the chimera is built; proven by tests, the content of anticancer substances (such as vinblastine, vincristine and vindoline) in a cell derived from the chimera is increased.
Owner:上海秋皇医药科技有限公司
Controlled release agent of containing fluorouracil and synergist
InactiveCN1957919AEasy injectionIncrease drug concentrationOrganic active ingredientsPeptide/protein ingredientsWhole bodyMicrosphere
A slowly-release anticancer medicine in the form of injection or implant is disclosed. Said slowly-release injection is composed of a special solvent containing suspending aid and the slowly-release microballs consisting anticance medicine, iterstitial hydrolyte and slowly-releasing auxiliary. Said anticancer medicine is chosen from 5-FU, vincristine, etc. Said interstitial hydrolyte is chosen from collagenase, relaxin, etc. Said slowly-releasing auxiliary is chosen from polylactic acid, FAD, polyethanediol, etc.
Owner:SHANDONG LANJIN PHARMA +1
Method for extracting and purifying vindoline
ActiveCN104370910AHigh yieldIncrease contentOrganic chemistryPhysical chemistryColumn chromatography
The invention discloses a method for extracting and purifying vindoline. The method includes: salting the whole plants of catharanthus roseus, freezing and drying, and crushing; adding the acquired powder into water, adjusting pH and then extracting, adjusting pH and then extracting the extract to obtain coarse vindoline; recrystallizing the coarse vindoline in mixed solvent to obtain pure vindoline. The method has the advantages that the method is simple in step, low in time consumption and fast in product separation, pH gradient separation is used to directly obtain the high-purity coarse vindoline while column chromatography isolation is not needed, the vindoline with the purity above 99% is acquired after recrystallization, purification is simplified, and separation and purification of the vindoline are achieved fast, and the obtained final product is high in yield and purity and capable of satisfying market requirements.
Owner:HAINAN XI YUAN CHEM TECH
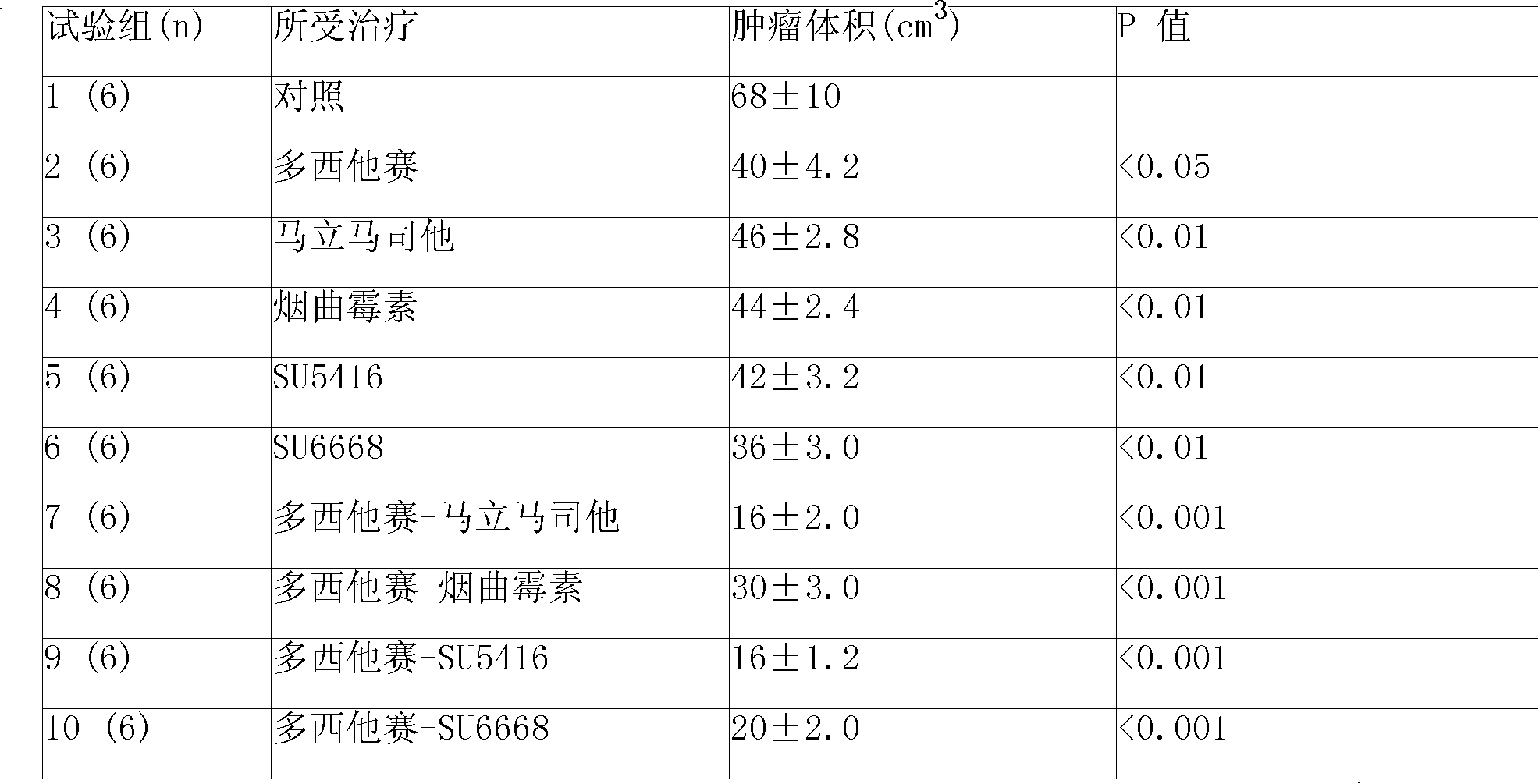
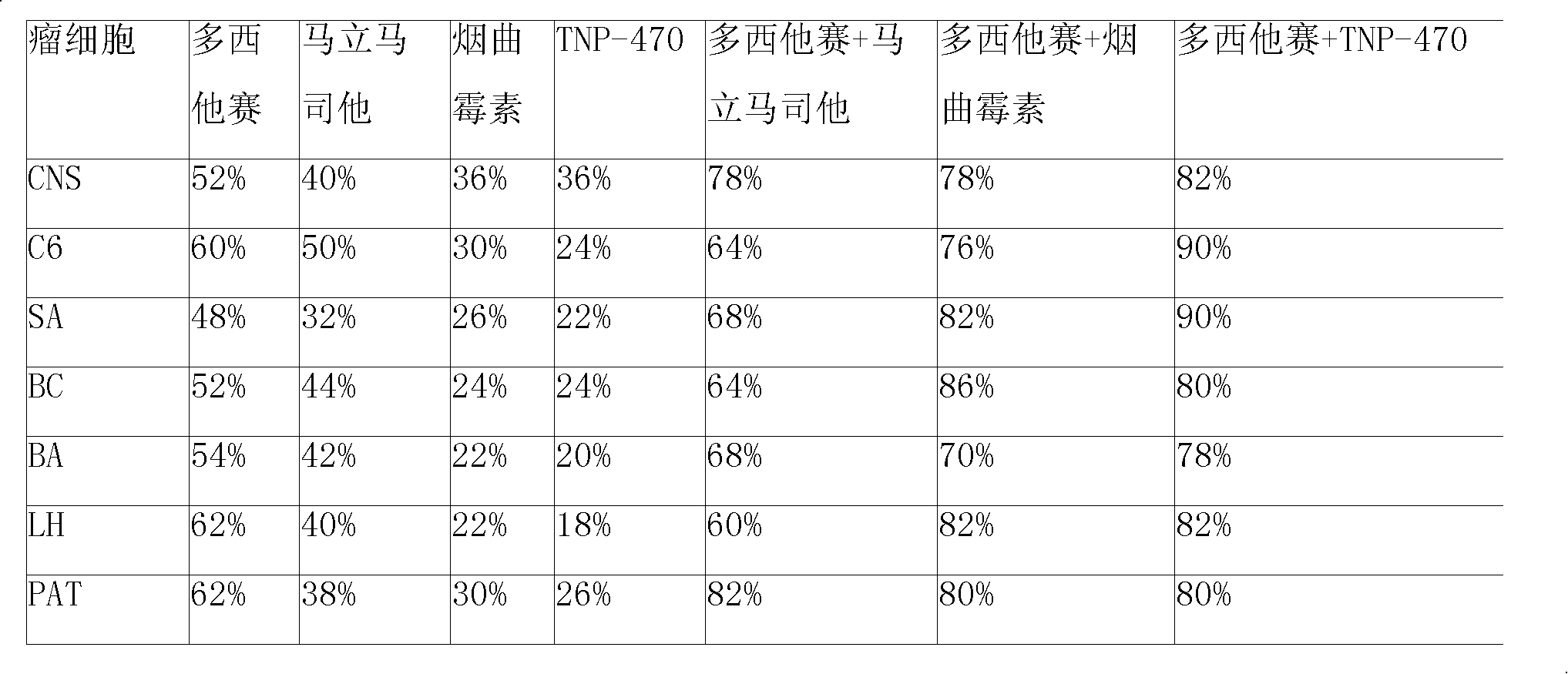
Compound sustained-released injection containing marimastat as neovascularization inhibitor
InactiveCN101336911APharmaceutical delivery mechanismPharmaceutical non-active ingredientsDepressantSuspending Agents
A compound sustained-released injection containing an angiogenesis inhibitor marimastat comprises sustained-released microspheres and a solvent. The sustained-released microspheres comprise a sustained-released adjuvant, an angiogenesis inhibitor selected from marimastat and fumagillin, and a cell toxicant selected from hydroxycamptothecin, mitozolomide, 4-carboxy temozolomide, docetaxel, oxaliplatin, sunplatinum, iphosphamide, lomustine, estramustine, fotemustine, semustine, etoposide, teniposide, vinblastine, anastrozole, fluorouracil and mitomycin c; and the solvent is a common solvent or a special solvent containing a suspending agent. The sustained-released adjuvant is selected from polifeprosan, poly(lactic acid), sebacic acid polymer such as poly(erucic acid dimmer-sebacic acid) and poly(fumaric acid-sebacic acid), EVAc, etc.; and the suspending agent has a viscosity of 100-3,000cp (20-30 DEG C) and is selected from sodium carboxymethyl cellulose, etc. The sustained-released microspheres can also be made into a sustained-released implant, which can enhance the curative effect of non-operative treatments such as chemotherapy and radiotherapy by intratumoral or peritumoral injection or placement.
Owner:JINAN KANGQUAN PHARMA TECH
Establishment method of chimeric cell culture system obtained by grafting periwinkle with periwinkle
The invention discloses a method for building a cell culture system for a chimera obtained through grafting vinca rosea to vinca minor. The method comprises the steps of cutting roots of the vinca minor, then, reserving 2 to 3 leaves for each vinca minor plant so as to prepare rootstock, inoculating growth points of the vinca rosea to the vinca minor rootstock under aseptic conditions, then, enabling each place of combination of a graft and the corresponding rootstock to emerge a callus, carrying out fluid suspension culture on the calli, and carrying out screening so as to obtain an excellent cell line. According to the method, after the growth points of the vinca rosea is grafted to the vinca minor, the calli are induced by using the obtained chimera as a material, and finally, an in-vitro cell suspension culture system for the chimera is built; proven by tests, the content of anticancer substances (such as vinblastine, vincristine and vindoline) in a cell derived from the chimera is increased.
Owner:上海秋皇医药科技有限公司
Compound sustained release injection containing newborn blood vessel inhibitor
InactiveCN101224191AOrganic active ingredientsPharmaceutical delivery mechanismDepressantSuspending Agents
The invention relates to a compound sustained-released injection containing a neovascularization inhibitor, which comprises sustained-release microspheres and menstruum; wherein, the sustained-release microspheres are composed of sustained-release excipients, the neovascularization inhibitor selected from marimastat or fumagillin, etc. and a cytotoxic drug selected from hydroxyl campto thecine, mitozolomide, 4-carboxyl temozolomide, docetaxel, oxaliplatin, hetaplatin, ifosfamide, lomustine, estramustine, fotemustine, samustine, etoposide, teniposide, vinblastine, anastrozole, fluorouracil or mitomycin C. The menstruum is special menstruum containing a suspending agent. The sustained-release excipients are selected from polifeprosan, polylactic acid, decanedioic acid polymer, such as poly (erucic acid dipolyme-decanedioic acid) and poly (allomaleic acid-decanedioic acid), etc. and EVAc, etc; the suspending agent, the viscosity of which is 100cp-3000cp (between 25 DEG C and 30 DEG C), is selected from carboxymethylcellulose sodium, etc. The sustained-release microspheres can also be prepared to be a sustained-release implant. The sustained-release implant is injected or deposited to the interior of tumour or around the tumour, which can improve the treatment effect of non-operative treatments, such as radio-chemotherapy, etc.
Owner:JINAN KANGQUAN PHARMA TECH
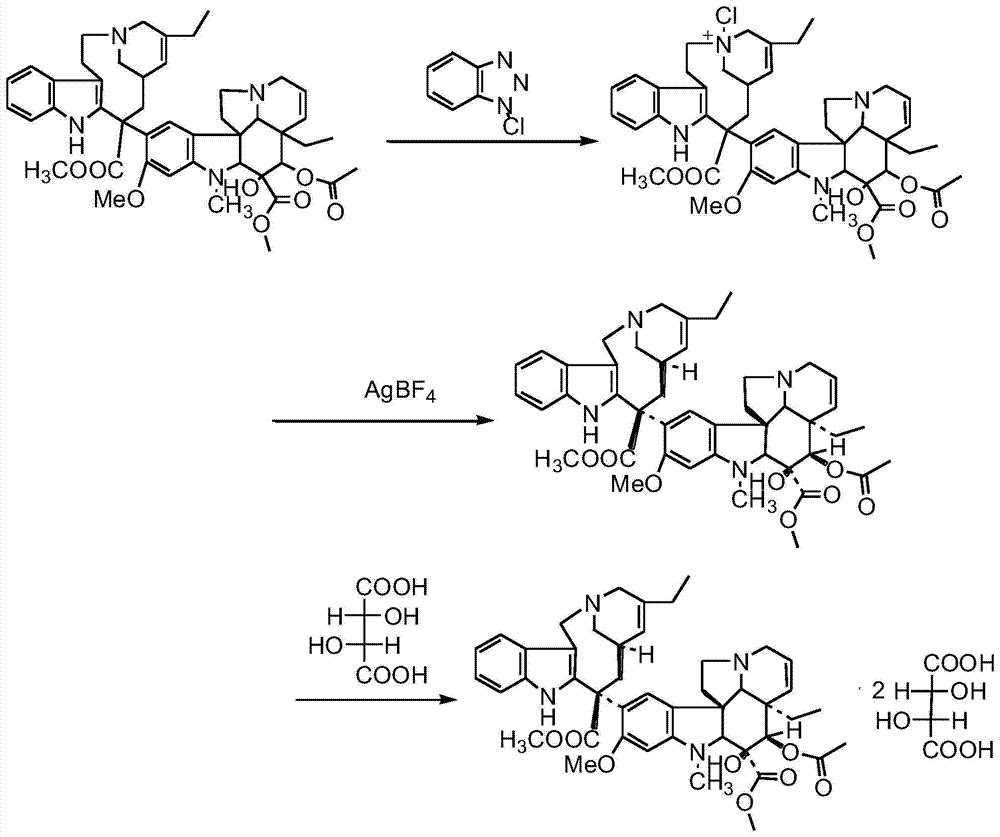
The preparation method of vinorelbine tartrate
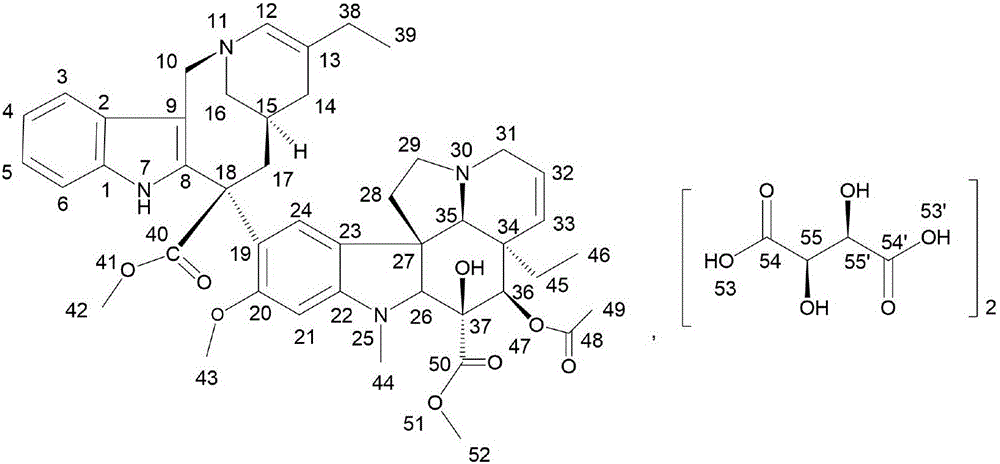
The invention relates to a preparation method of vinorelbine tartrate. The preparation method comprises the following steps: oxidizing a compound as shown in the formula I by the use of iron chloride and organic peracid, coupling by adding vindoline, carrying out a reduction reaction by adding a reducing agent, letting the reduction reaction product react with L-tartaric acid to form salt so as to prepare vinorelbine tartrate. According to the product obtained by the preparation method, yield is high, and purity and chiral purity (ee value) are very good.
Owner:JIANGSU HANSOH PHARMA CO LTD
Anticancer slow release preparation of nimustine and its progression agent
InactiveCN1919179AEasy injectionIncreased sensitivityOrganic active ingredientsPeptide/protein ingredientsTreatment effectWhole body
Disclosed is slow release anticancer agent carrying both Nimustine and its synergistic agents, which comprises slow release auxiliary materials and active anticancer constituents, wherein the active anticancer constituents include Nimustine and its synergistic agents (such as Vinblastine, Goserelin or Carmofur). The viscosity of the slow release injection is 10-650cp (at 20-30 deg C), and the active anticancer constituents can also be made into slow release implanting agent. The slow release subsidiary materials mainly comprise bio-compactable and degradable macromolecular polymers, when locally dispensed on the tumor, the composition not only can lower down the whole body toxicity reaction of the anti-cancer medicament, but also can selectively increase the tumor local medicinal concentration, the treatment effect of the non-operative treatment methods such as chemotherapy, medicament and radiation can also be improved. The solid tumors include glioma, osteosarcoma, lymphoma, lung carcinoma, intestinal cancer, and breast cancer.
Owner:JINAN KANGQUAN PHARMA TECH
Functionalized vinblastine liposome and its application
ActiveCN107875123BOrganic active ingredientsMacromolecular non-active ingredientsPolyethylene glycolLiposome
Owner:PEKING UNIV
Preparation method of vinorelbine tartrate
The invention relates to a preparation method of vinorelbine tartrate. The preparation method comprises the following steps: oxidizing a compound as shown in the formula I by the use of iron chloride and organic peracid, coupling by adding vindoline, carrying out a reduction reaction by adding a reducing agent, letting the reduction reaction product react with L-tartaric acid to form salt so as to prepare vinorelbine tartrate. According to the product obtained by the preparation method, yield is high, and purity and chiral purity (ee value) are very good.
Owner:JIANGSU HANSOH PHARMA CO LTD
Method for producing vindoline
The invention discloses a method for producing vindoline. The method comprises: inoculating a periwinkle polyploid cell strain in a synthetic medium, and obtaining a cell rich in vindoline by cultivating the strain for 3 to 7 days at the temperature between 20 and 28DEG C; and the synthetic medium is a liquid culture medium obtained by adding 0 to 4mg / L of plant cell auxin, 0 to 4mg / L of plant cytokinin, 30 to 60g / L of sucrose, 10 to 40mu g / L of acetyl coenzyme A, 0.5 to 1.5mu mol / L of Benzotriazole methl, 5 to 30mu mol / L of tranylcypromine, 10 to 40mu l / L of acetic anhydride and 10 to 40mg / Lof dithiothreitol in a minimal medium. The method using periwinkle cells to cultivate and produce vindoline can stably realize the industrialized production of vindoline, and the production cost is rather low, the culture period is short, and the production is insusceptible to the natural environment and weather, so the year-round production can be realized.
Owner:TSINGHUA UNIV
A compound sustained release anticancer agent containing angiogenesis inhibitor
Disclosed is an anticancer slow release injection containing anti-angiogenesis, which comprises slow release micro-balloons and dissolvent, wherein the slow release micro-balloons comprises slow release auxiliary materials and anti-angiogenesis selected from Marimastat or fungillin, and cytotoxic drugs selected from hydroxycamptothecine, Mitozolomide, 4-carboxyl temozolomide, docetaxel, Oxaliplatin, Eptaplatin, Ifosfamide, Lomustine, Estramustine, Fotemustine, Semustine, Etoposide, Teniposide, Vinblastine, Anastrozole, fluorouracil or Mitomycin C, the dissolvent being specific dissolvent containing suspension adjuvant. The slow release auxiliary materials are selected from Polifeprosan, poly(lactic acid), poly(erucic aciddipolymer-sebacylic acid) and poly(fumaric acid-sebacylic acid), and EVAc. The viscosity of the suspension adjuvant is 100-3000cp (at 25-30 deg C), and is selected from sodium carboxymethylcellulose.
Owner:JINAN KANGQUAN PHARMA TECH
Compound sustained release injection containing newborn blood vessel inhibitor fumagillin
InactiveCN101224190AOrganic active ingredientsPharmaceutical delivery mechanismDepressantTherapeutic effect
The invention relates to a compound sustained-released injection containing a neovascularization inhibitor, which comprises sustained-release microspheres and menstruum; wherein, the sustained-release microspheres are composed of sustained-release excipients, the neovascularization inhibitor selected from marimastat or fumagillin, etc. and a cytotoxic drug selected from hydroxyl campto thecine, mitozolomide, 4-carboxyl temozolomide, docetaxel, oxaliplatin, hetaplatin, ifosfamide, lomustine, estramustine, fotemustine, samustine, etoposide, teniposide, vinblastine, anastrozole, fluorouracil or mitomycin C. The menstruum is special menstruum containing a suspending agent. The sustained-release excipients are selected from polifeprosan, polylactic acid, decanedioic acid polymer, such as poly (erucic acid dipolyme-decanedioic acid) and poly (allomaleic acid-decanedioic acid), etc. and EVAc, etc; the suspending agent, the viscosity of which is 100cp-3000cp (between 25 DEG C and 30 DEG C), is selected from carboxymethylcellulose sodium, etc. The sustained-release microspheres can also be prepared to be a sustained-release implant. The sustained-release implant is injected or deposited to the interior of tumour or around the tumour, which can improve the treatment effect of non-operative treatments, such as radio-chemotherapy, etc.
Owner:JINAN KANGQUAN PHARMA TECH
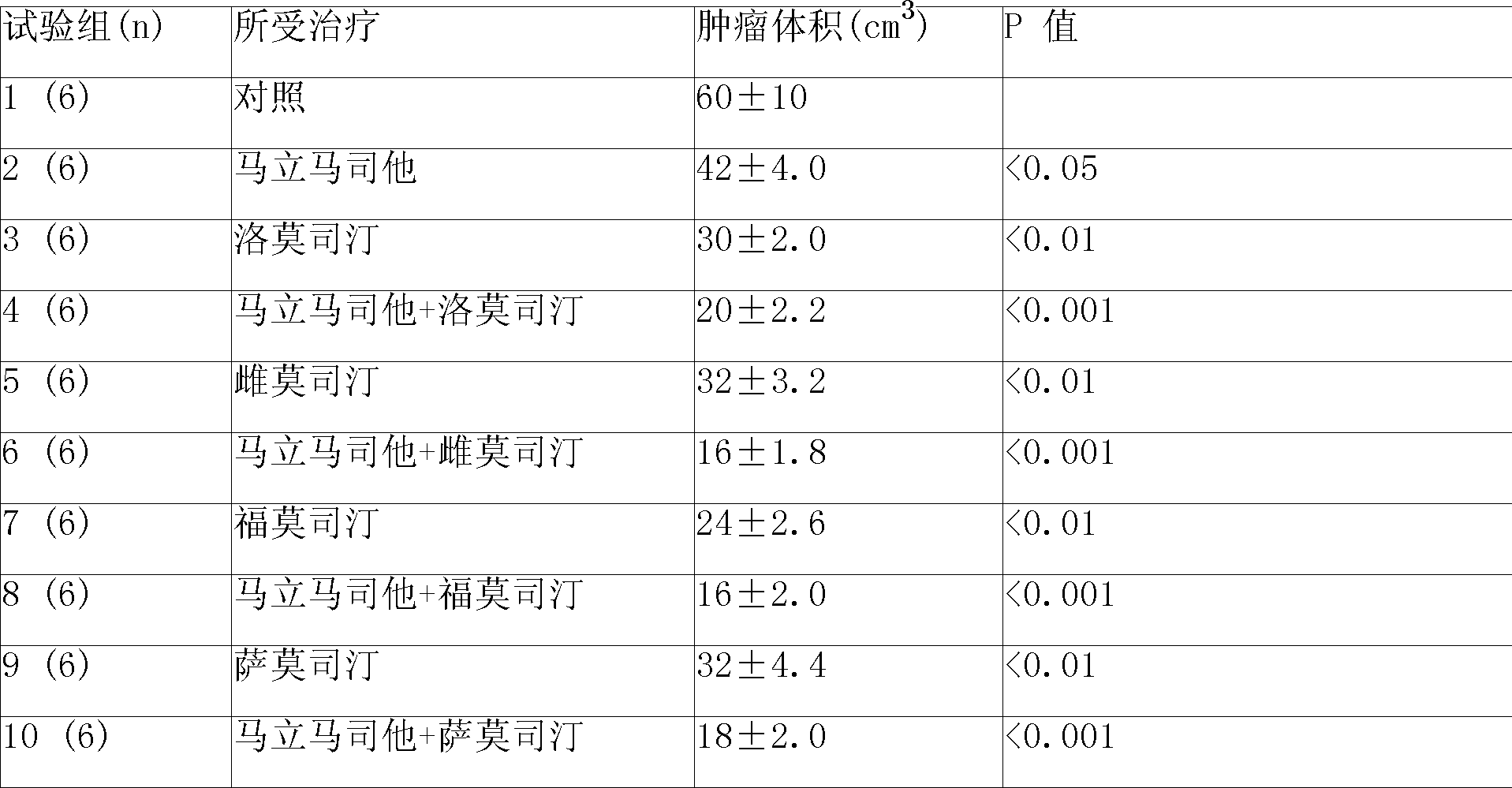
Anti-cancer composition loading both mtrosourea medicament and alkaloids medicament
InactiveCN101011348APharmaceutical delivery mechanismPharmaceutical non-active ingredientsAdjuvantTreatment effect
Disclosed is a slow release injection agent of anticancer composition containing nitrosourea drugs and alkaloid drugs, which comprises slow release microspheres and dissolvent, wherein the slow release microspheres comprise anti-cancer active constituents and slow release auxiliary materials, the dissolvent being conventional dissolvent or specific dissolvent containing suspension adjuvant. The viscosity of the suspension adjuvant is 100-3000cp (at 20-30 deg C), and is selected from sodium carboxymethylcellulose, the nitrosourea drugs are selected from Carmustine, Nimustine, Fotemustine, Lomustine or Bendamustine, the alkaloid drugs include Vincristine, Vinblastine, Vinorelbine, Vindesine and Vinrosidine, the slow release auxiliary materials are selected from polyphosphate ester copolymers such as p(LAEG-EOP), p(DAPG-EOP), copolymer or blend of polyphosphate ester with polylactic acid, Polifeprosan, sebacylic acid and PLGA. The anticancer composition can also be prepared into slow release implanting agent, for injection or placement in or around tumor with a period of effective concentration maintenance over 60 days, as well as the treatment effect of appreciably lowering general reaction of the drugs, and improving the treatment effect of the non-operative treatment methods such as chemotherapy.
Owner:JINAN SHUAIHUA PHARMA TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com