Preparation method of vinorelbine tartrate
A technology of vinorelbine tartrate and tartaric acid, which is applied in the field of medicine and can solve problems affecting the clarity, stability, and safety of preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
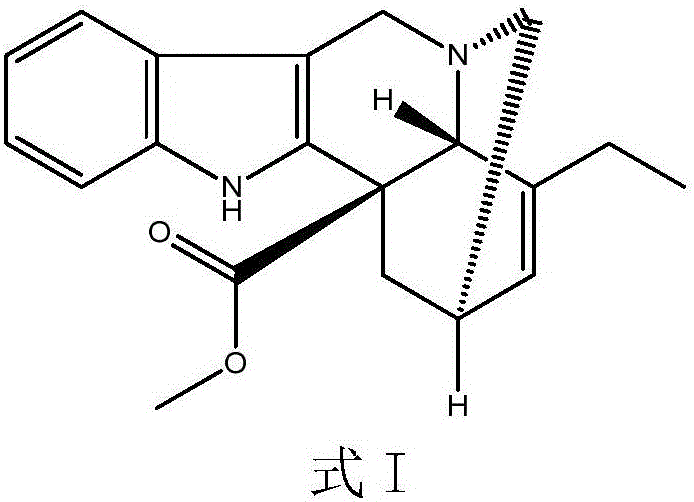
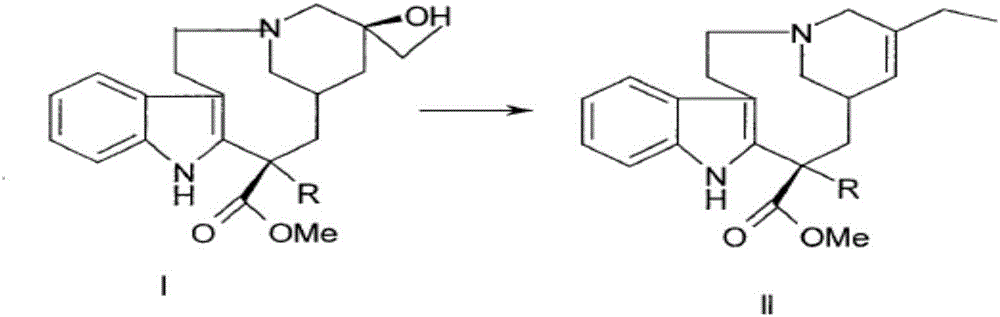
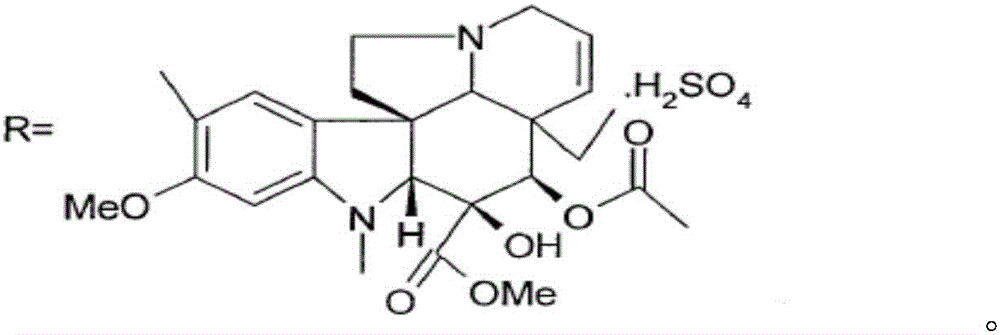
[0031] Add 10mmol of the compound of formula I to 100ml of trifluoroethanol, control the temperature to 0°C, add 10ml of 3mol / L sodium chloride solution of ferric chloride dropwise under stirring, add 10mmol of peracetic acid, and stir at 0°C for reaction 2h, then add 11mmol of ventolin, continue to stir and react at 0°C for 4h; control the temperature to 75°C, continue to add 100ml of 2mol / L sodium acetate solution, then add 40mmol of thiourea dioxide, react at 75°C for 3h, and use dichloromethane Extract with 100mL×3, wash with saturated saline, dry and evaporate the solvent under reduced pressure; continue to add 150ml of acetone and 15mmol of L-tartaric acid to react to form a salt, add anhydrous ether dropwise under stirring until the precipitate is fully separated out, filter, and dry in vacuum to obtain pure white The product is 9.59g, and the product obtained through testing is vinorelbine tartrate (see the attached form for identification data), the product purity is 9...
Embodiment 2
[0043] Add 10mmol of the compound of formula I to 100ml of trifluoroethanol, control the temperature to 0°C, add dropwise 10ml of 3mol / L sodium chloride solution of ferric chloride with stirring, add 10mmol of peroxybenzoic acid, and stir at 0°C React for 2 hours, then add 11 mmol of ventolin, continue to stir the reaction for 4 hours at 0°C; Extract with 100mL×3 methane, wash with saturated brine, dry and evaporate the solvent under reduced pressure; continue to add 150ml of acetone and 15mmol of L-tartaric acid to react to form a salt, add anhydrous ether dropwise under stirring until the precipitate is fully separated, filtered, and dried in vacuo to obtain pure White product 9.67g. 13 The CNMR spectrum is consistent with Table 1 and Table 2, and the detected purity is 95.0%, the ee value is 99.8%, and the molar yield is 85% (based on the compound of formula I).
Embodiment 3
[0045] Add 10mmol of the compound of formula I to 100ml of trifluoroethanol, control the temperature to 0°C, add dropwise 10ml of 3mol / L sodium chloride solution of ferric chloride with stirring, add 10mmol of peroxybenzoic acid, and stir at 0°C React for 2 hours, then add 11 mmol of ventolin, continue to stir the reaction at 0°C for 4 hours; Extract with 100mL×3 methane, wash with saturated brine, dry and evaporate the solvent under reduced pressure; continue to add 150ml of acetone and 15mmol of L-tartaric acid to react to form a salt, add anhydrous ether dropwise under stirring until the precipitate is fully separated, filtered, and dried in vacuo to obtain pure White product 9.78g. 13 The CNMR spectrum is consistent with Table 1 and Table 2, and the detected purity is 99.5%, the ee value is 99.8%, and the molar yield is 90% (based on the compound of formula I).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com