Preparation method of vinorelbine
A technology of vinorelbine and vinorelbine tartrate, which is applied in the synthesis field of the antitumor compound vinorelbine tartrate, can solve the problems of low purity, difference, different substituent modification methods, etc., and achieves the effect of high purity and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
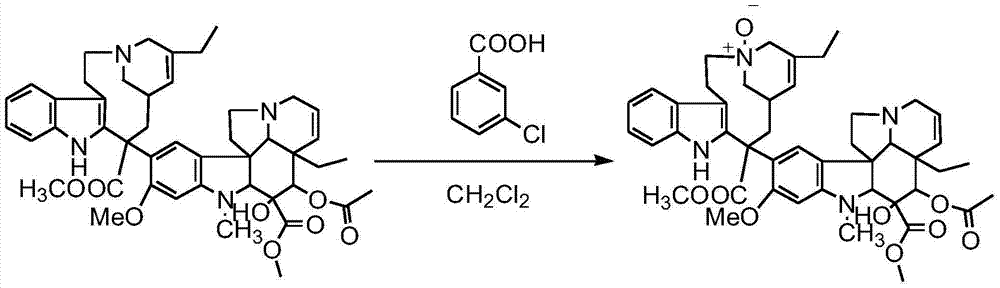
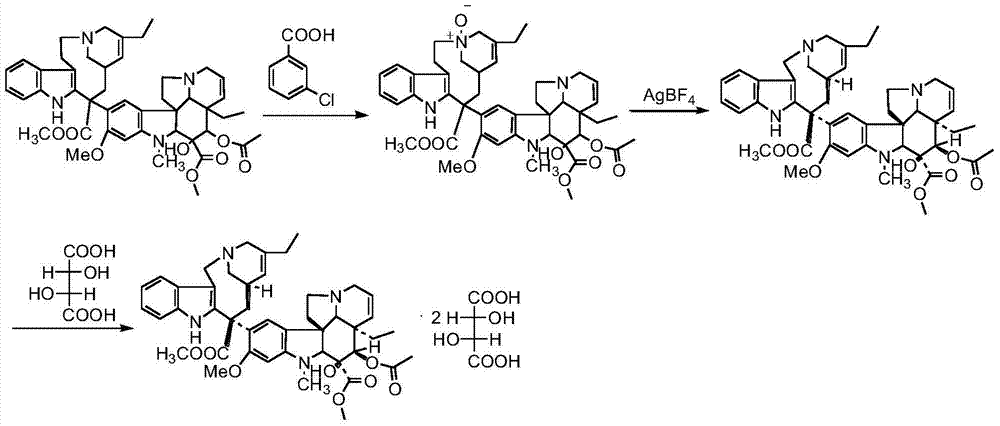
[0047] Under dark conditions, pass N 2 Exhaust the air in the 50L reactor, add 10.8L of distilled water into the reaction flask, and then add 67.00g of sodium chloride, 85.00g of glycine, 640.00g of ferric chloride hexahydrate, 200.00g of vinblastine tartrate, 200.00g of Wenduo Dissolve Ling with 10.5L 0.1N hydrochloric acid aqueous solution and add it to the reaction kettle. After stirring at room temperature for 24 hours, dissolve 22.00g of sodium borohydride in 2.5L of frozen ammonia water and add it dropwise to the reaction solution with a constant pressure dropping funnel, and drop it in about 30min. After the addition is complete, continue stirring at room temperature for 30 minutes to stop the reaction, extract three times with 3500ml, 3000ml, and 3000ml of dichloromethane successively, combine the organic phases and add 200.00g of anhydrous sodium sulfate to dry for 10min, and then use a sand with 350.00g of diatomaceous earth Filter the dry liquid through a core funne...
Embodiment 2
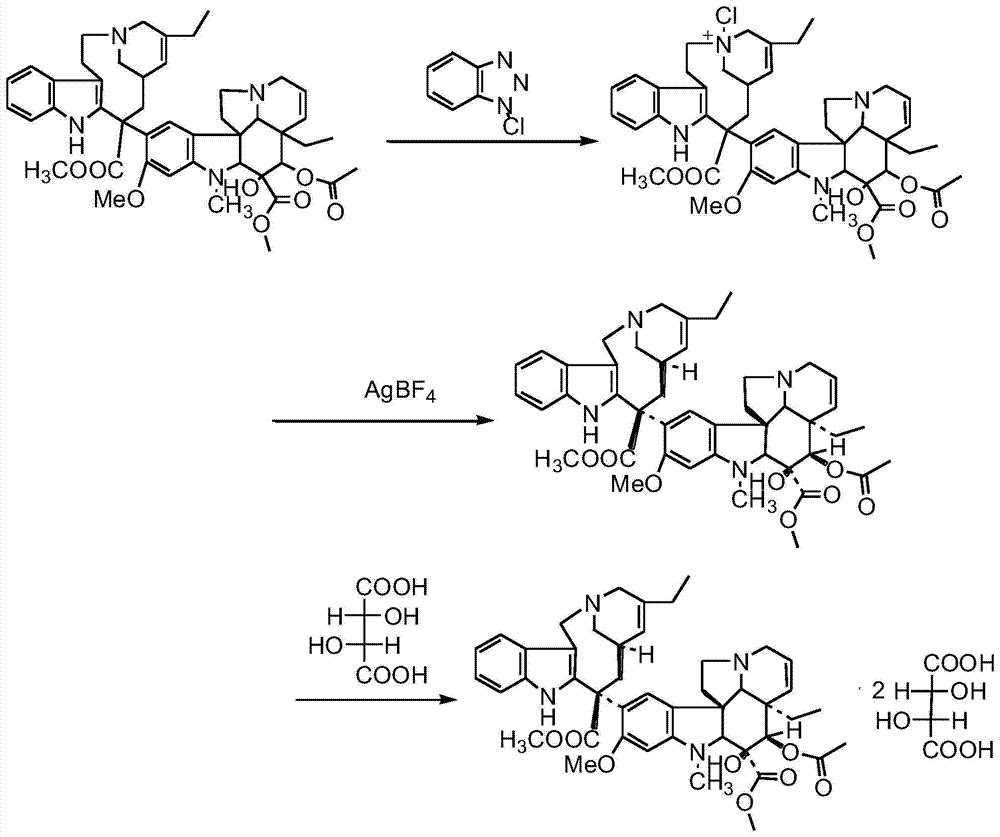
[0049] Under dark conditions, pass N 2 Exhaust the air in the 10L reaction flask, add 240.00g of dehydrated vinblastine to the 10L reaction flask, then add 940ml of dichloromethane, stir to dissolve, adjust the dry ice-acetone bath temperature to -65~-60°C and maintain it for 20min, add 60.00 g NBS and 40ml of trifluoroacetic acid were dissolved in 410ml of dichloromethane and added dropwise to the reaction solution using a constant pressure dropping funnel. The dropwise addition was completed in about 30 minutes. Continue to control the temperature and stir the reaction at -65~-60°C for 2 hours, and then add 65.00g Dissolve ammonium acetate in 360ml of distilled water and add the reaction liquid, replace the acetone bath at -65~-60℃ with the acetone bath at -30~-20℃ and continue stirring for 10min, add 520ml tetrahydrofuran and 520ml distilled water to dissolve 68.00g silver tetrafluoroborate Remove the -30~-20°C acetone bath and stir the reaction at room temperature for 24h,...
Embodiment 3
[0051] Normal phase silica gel column purification:
[0052] Preparation of saturated methanol-ammonia gas solution: Introduce ammonia gas into the anhydrous methanol solution for about 3 hours to reach saturation.
[0053] Preparation of mobile phase solution: Take 2000ml of methanol-ammonia solution just prepared and add it to 80L of dichloromethane and mix well.
[0054] Column loading: In the dark, weigh 8000g of 400-500 mesh silica gel with 40L of mobile phase and stir evenly into the column, and close the valve when the liquid level is 2-5mm higher than the interface.
[0055] Sample loading: 260.00 g of crude vinorelbine was dissolved in 260 ml of mobile phase, and added to the column.
[0056] Elution: add mobile phase, control the flow rate to 3ml / min, start elution, collect them separately in 500ml brown bottles, monitor by TLC, combine the target eluent, concentrate under reduced pressure to dryness, pump with oil pump for 24h to obtain constant weight Target 105....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com