Preparation method for vinorelbine tartrate
A technology of vinorelbine tartrate and vinorelbine, which is applied in the field of preparation of vinorelbine tartrate, can solve the problems of high price of dehydrated vinblastine, high industrial cost, and difficult control, and achieve easy control of the operation process, low cost, The effect of less side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
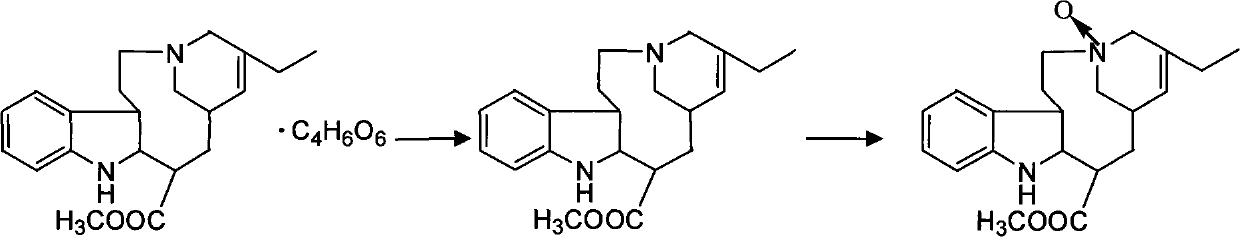
[0035] (1) Synthesis of vinblastine-N oxide
[0036] Take 70g of vinblastine tartrate, put it in a three-necked flask, add 2L of distilled water, stir to make it completely dissolved, then add 350ml of ammonia water and stir, extract with dichloromethane, separate the organic layer, take 23g of m-chloroperoxybenzoic acid and slowly add In the above-mentioned organic layer, after stirring at room temperature until the reaction is complete, add 1300ml 10% sodium carbonate solution (the purpose of adding sodium carbonate solution is to make the precipitation completely separate out, generally get final product with 5%~20% sodium carbonate solution), Stir, filter, and vacuum-dry to obtain 38 g of vinblastine-N oxide with a purity of 98.6% and a yield of 54% (calculated as vinblastine tartrate).
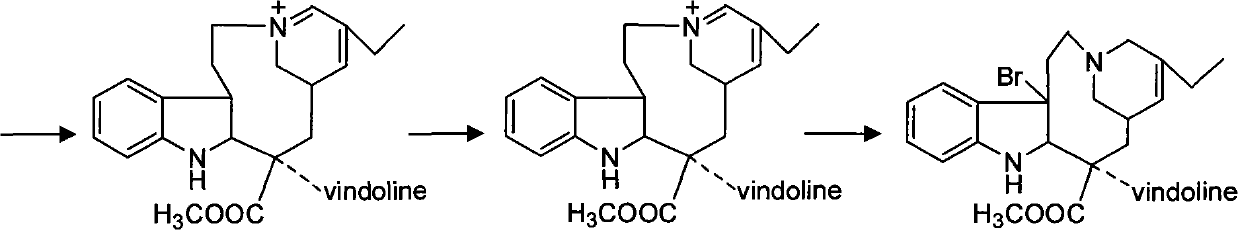
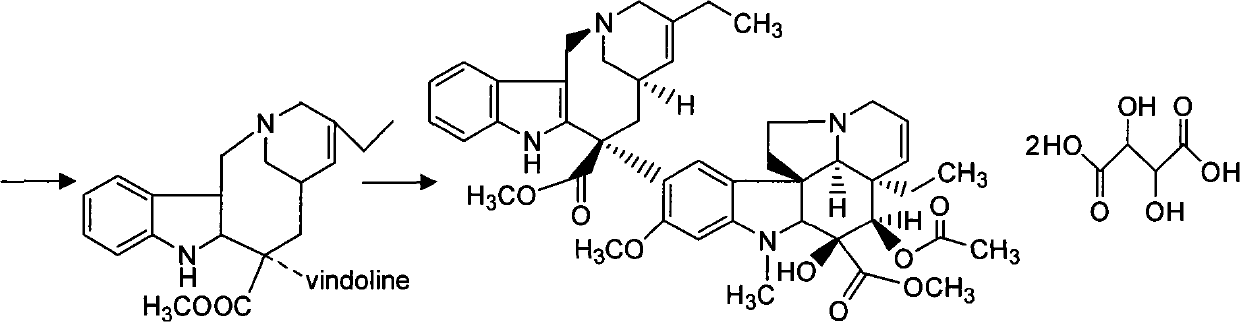
[0037] (2) Synthesis of Dehydrovinblastine
[0038]Get the vinblastine-N oxide 38g and 49.0g Wenduolin that step (1) makes, put in the three-necked reaction bottle, add methylene chlorid...
Embodiment 2
[0076] (1) Synthesis of vinblastine-N oxide
[0077] Take 68g of vinblastine tartrate, put it in a three-necked flask, add 2L of distilled water, stir to make it completely dissolved, then add 350ml of ammonia water and stir, extract with dichloromethane, separate the organic layer, take 22g of m-chloroperoxybenzoic acid and slowly add In the above organic layer, after stirring at room temperature to complete the reaction, add 1500ml of 5% sodium carbonate solution, stir, filter, and vacuum-dry to obtain 37.5g of vinblastine-N oxide with a purity of 98.8% and a yield of 55%. (calculated as vinblastine tartrate).
[0078] (2) Synthesis of Dehydrovinblastine
[0079] Get vinblastine-N oxide 37.5g and 49.2g pendolin that step (1) makes, put in the three-necked reaction bottle, add 400ml of dichloromethane, stir and fully react at room temperature, then move into the ultra-low temperature cold bath, Observe that the temperature in the bottle reaches -91°C, slowly add 60ml of tri...
Embodiment 3
[0085] (1) Synthesis of vinblastine-N oxide
[0086] Take 72g of vinblastine tartrate, put it in a three-necked flask, add 2L of distilled water, stir to make it completely dissolved, then add 350ml of ammonia water and stir, extract with dichloromethane, separate the organic layer, take 25g of m-chloroperoxybenzoic acid and slowly add In the above-mentioned organic layer, after stirring and reacting completely at room temperature, add 1000ml 20% sodium carbonate solution, stir, filter, vacuum-dry, obtain 40g vinblastine-N oxide compound, purity 98.6%, yield is 55.6% (with vinblastine tartrate).
[0087] (2) Synthesis of Dehydrovinblastine
[0088]Get 40g of vinblastine-N oxide and 51g of Vindolin prepared in step (1), put them in a three-necked reaction bottle, add 400ml of dichloromethane, stir and fully react at room temperature, then move into an ultra-low temperature cold bath, observe the bottle When the internal temperature reaches -90°C, slowly add 65ml of trifluoroa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com