Preparation method of thioether compound and product of preparation method
A compound and thioether technology, applied in the preparation of thioethers, steroids, organic chemistry, etc., can solve the problems of large amount of metal used, high temperature, long reaction time, etc., to eliminate metal residues, high yield, product good biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]
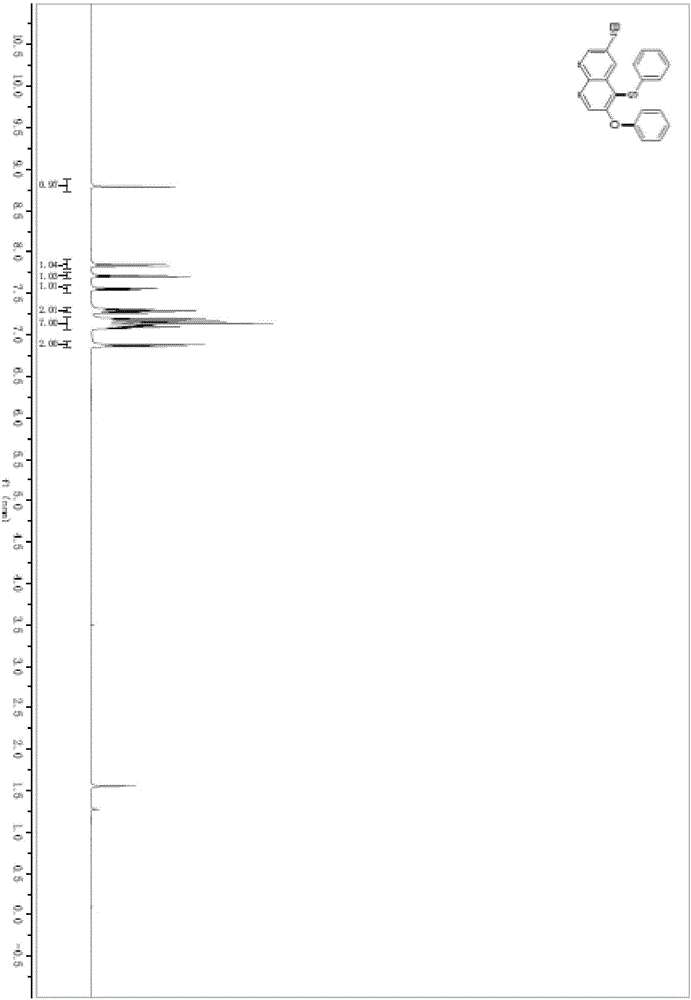
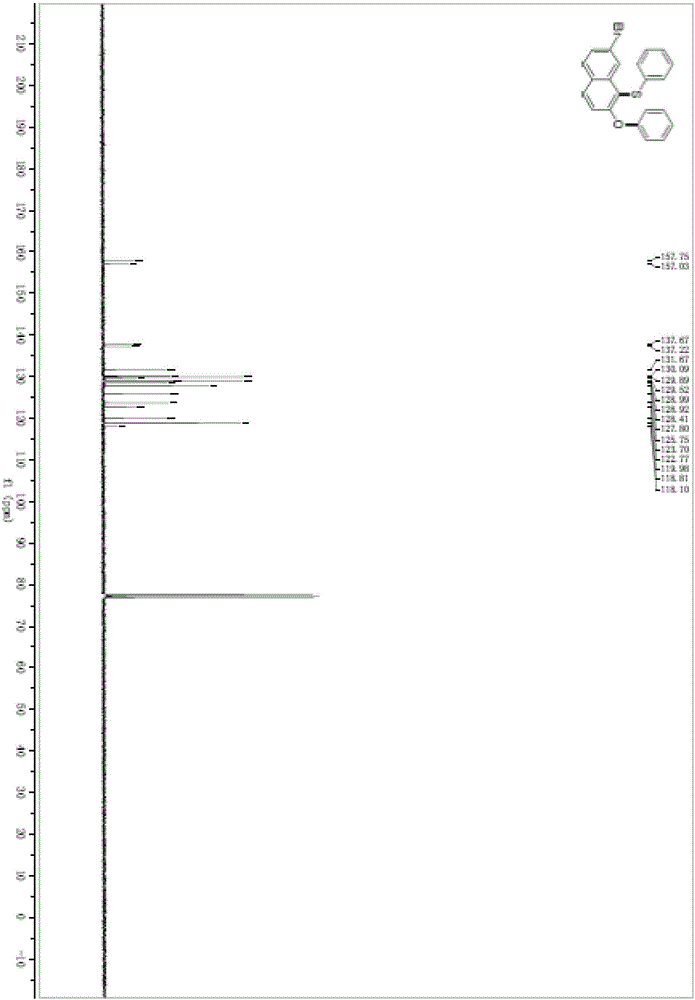
[0045] In a pre-dried 5mL round-bottomed flask, under the protection of argon, add acetonitrile (2mL) and diphenylsulfoxide (44.5mg, 0.22mmol) successively, cool down to 0°C, and slowly add trifluoromethanesulfonic anhydride (40.8μL , 0.24mmol), added 2-hydroxy-7-bromonaphthalene (44.6mg, 0.2mmol) and stirred at 500rpm at this temperature for 3h. Potassium phosphate (188.7 mg, 0.44 mmol) was added, heated to 70° C., and stirred at 500 rpm for 24 hours.
[0046] After the reaction solution was stirred, it was washed with water, extracted three times with dichloromethane, and the organic phases extracted several times were combined into a 100mL eggplant-shaped flask, using a Heidolph rotary evaporator with a rotation speed of 120rpm, a temperature of 38°C, and a vacuum of 0.1Mpa , treated for 3 min, and then performed column chromatography using 200-mesh column chromatography silica gel, and the developing solvent was petroleum ether:ethyl acetate=99:1, and the targe...
Embodiment 2
[0050]
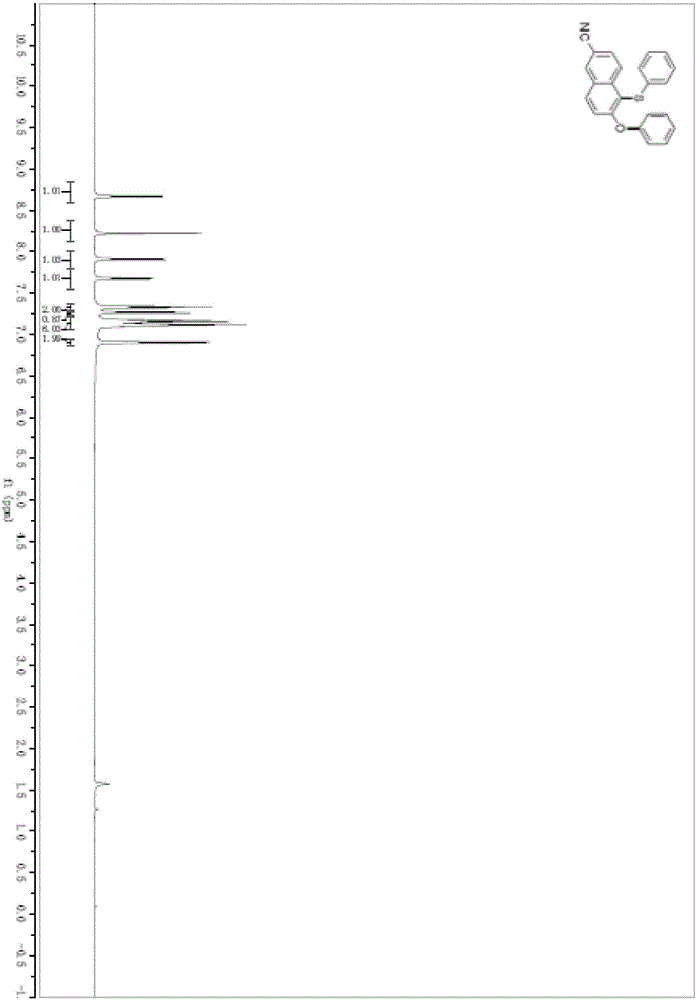
[0051] In a pre-dried 5mL round-bottomed flask, under the protection of argon, add acetonitrile (2mL) and diphenylsulfoxide (44.5mg, 0.22mmol) successively, cool down to 0°C, and slowly add trifluoromethanesulfonic anhydride (40.8μL , 0.24mmol), added 2-hydroxy-6-cyanonaphthalene (33.9mg, 0.2mmol), stirred at 600rpm at this temperature for 3h. Potassium phosphate (188.7 mg, 0.88 mmol) was added, heated to 70° C., and stirred at 600 rpm for 24 hours.
[0052] After the reaction solution was stirred, it was washed with water, extracted three times with dichloromethane, and the organic phases extracted several times were combined into a 100mL eggplant-shaped flask, using a Heidolph rotary evaporator with a rotation speed of 200rpm, a temperature of 38°C, and a vacuum of 0.1Mpa , treated for 4min, and then carried out column chromatography using 200-mesh column chromatography silica gel, and the developer was petroleum ether:ethyl acetate=99:1, and the target compound ...
Embodiment 3
[0056]
[0057] In a pre-dried 5mL round bottom flask, under the protection of argon, add acetonitrile (2mL), 4-methylphenyl-n-hexyl sulfoxide (49.4mg, 0.22mmol) successively, cool down to 0°C, and slowly add trifluoro Add methanesulfonic anhydride (40.8 μL, 0.24 mmol), add 2-hydroxynaphthalene (28.9 mg, 0.2 mmol), and stir at 700 rpm for three hours at this temperature. Potassium phosphate (188.7 mg, 0.88 mmol) was added, heated to 70° C., and stirred at 700 rpm for 24 hours.
[0058] After the reaction solution was stirred, it was washed with water, extracted three times with dichloromethane, and the organic phases extracted several times were combined into a 100mL eggplant-shaped flask, using a Heidolph rotary evaporator with a rotation speed of 200rpm, a temperature of 38°C, and a vacuum of 0.1Mpa , the treatment time was 3min, and then 200 mesh column chromatography silica gel was used for column chromatography, and the developer was petroleum ether:ethyl acetate=99:1,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com