Preparation method for synthesizing polysubstituted cyclic 1,2-diketone by sulfoxide participating in arylation
A multi-substitution and chemical synthesis technology, applied in the preparation of sulfides, organic chemistry, etc., can solve the problems of expensive transition metals, high reaction temperature, etc., and achieve the effect of eliminating metal residues, wide substrate range, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
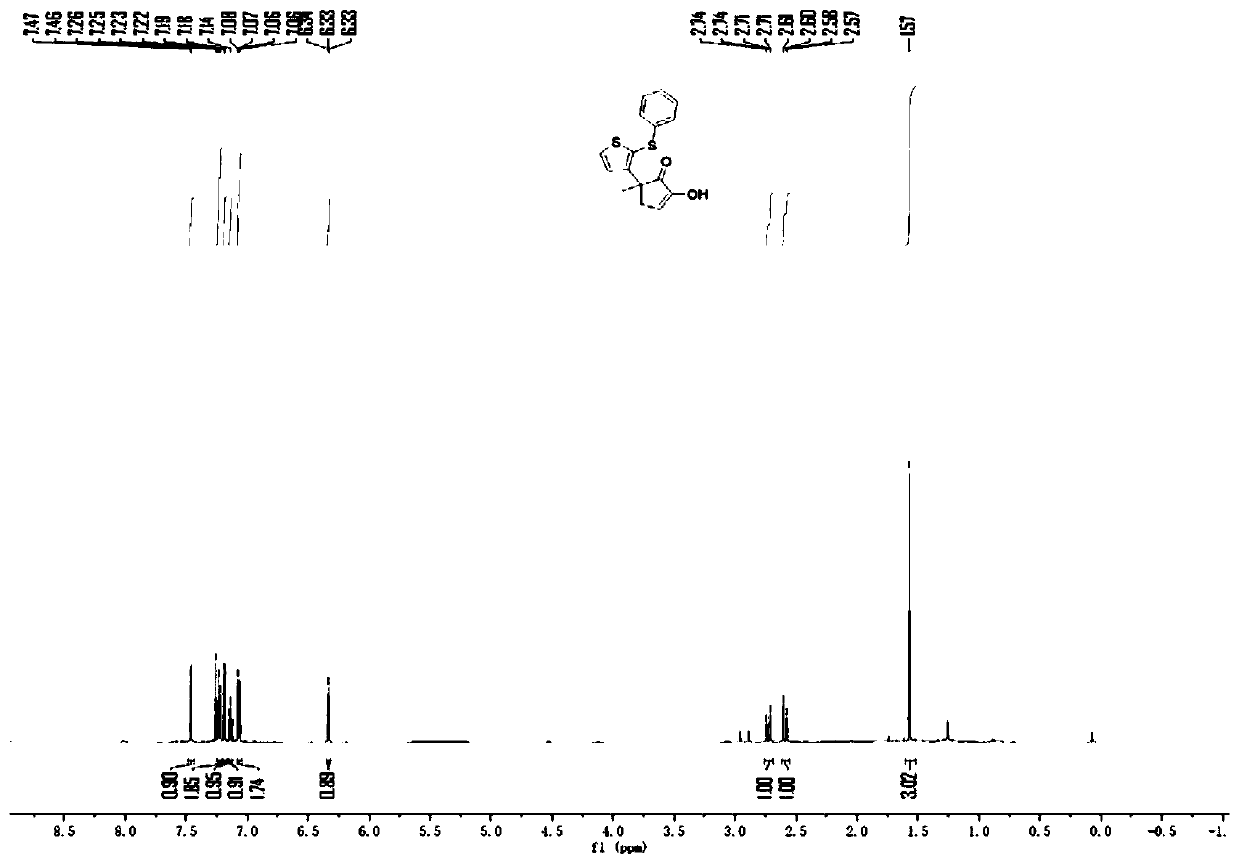
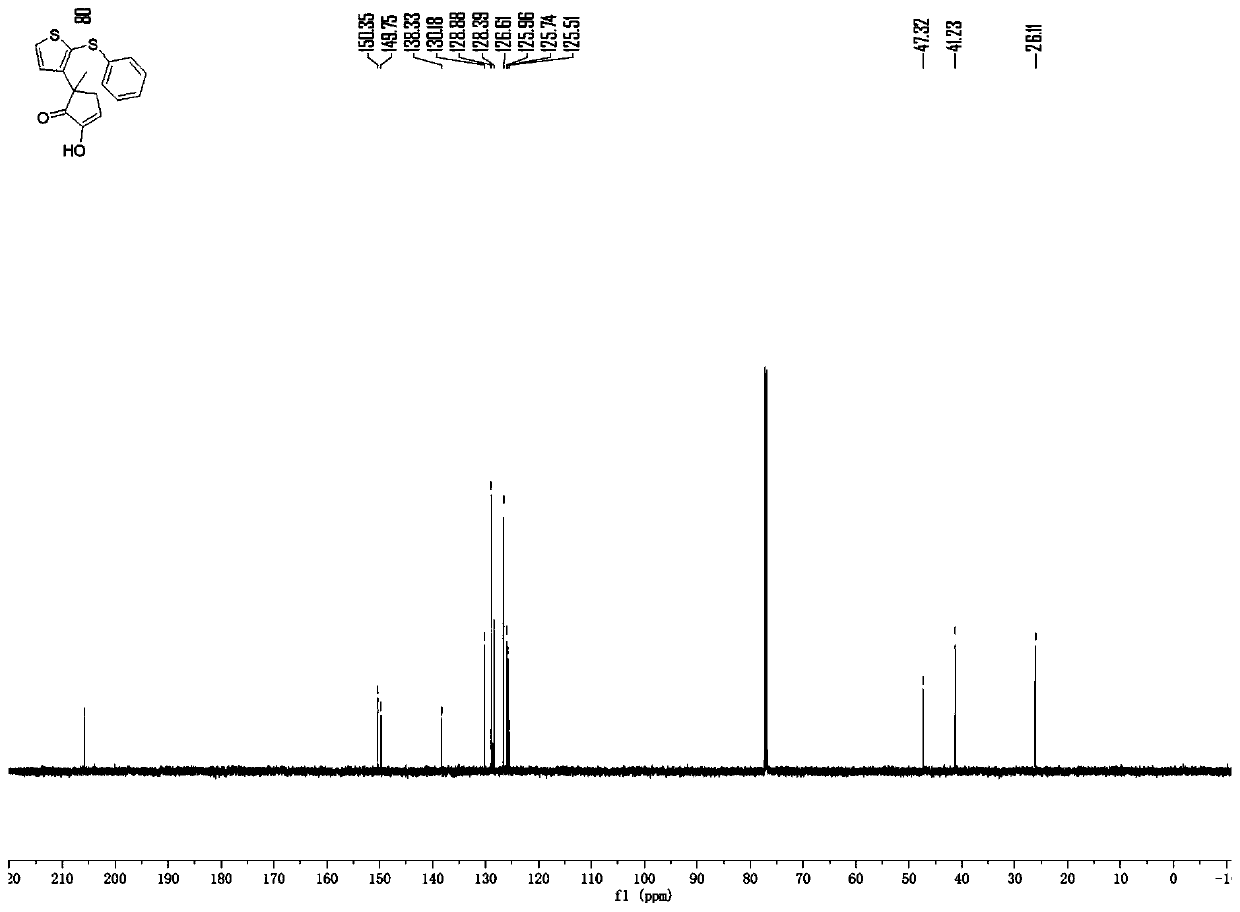
[0036]
[0037] To a 20mL test tube, add phenylthiophene sulfoxide (21mg, 0.1mmol) and 3-methylcyclopentane-1,2dione (17.1mg, 0.15mmol) successively at room temperature, and add 0.8ml of dichloro Ethane, placed in a low-temperature reactor at -10°C, wait for the reaction system to mix evenly and the reactant body will be -10°C, add trifluoroacetic anhydride (21mg, 0.15mmol), and react at a low temperature of -10°C for 4h, then The reaction mixture was allowed to warm to room temperature (typically 25°C) and stirred at this temperature for 1 min. After completion, the reaction system in the test tube was transferred to a 10ml eggplant-shaped bottle without any post-processing operation. Using a Heidolph rotary evaporator, the rotation speed was 80-100rpm, the temperature was 38°C, and the vacuum degree was 0.1Mpa. The residue was subjected to column chromatography using 200-mesh column chromatography silica gel, and the developer was petroleum ether:ethyl acetate=9:1, and th...
Embodiment 2
[0041]
[0042] To a 20mL test tube, diphenylsulfoxide (20.6mg, 0.1mmol) and 3-methylcyclopentane-1,2dione (17.1mg, 0.15mmol) were added sequentially at room temperature, and 0.8ml di Ethyl chloride, placed in a low-temperature reactor at -10°C, wait for the reaction system to mix evenly and the reaction body will be -10°C, add trifluoroacetic anhydride (21mg, 0.15mmol), and react at a low temperature of -10°C for 4h, The reaction mixture was then allowed to warm to room temperature (typically 25°C) and stirred at this temperature for 1 min. After completion, the reaction system in the test tube was transferred to a 10ml eggplant-shaped bottle without any post-processing operation. Using a Heidolph rotary evaporator, the rotation speed was 80-100rpm, the temperature was 38°C, and the vacuum degree was 0.1Mpa. The residue was subjected to column chromatography using 200-mesh column chromatography silica gel, and the developer was petroleum ether:ethyl acetate=9:1, and the ta...
Embodiment 3
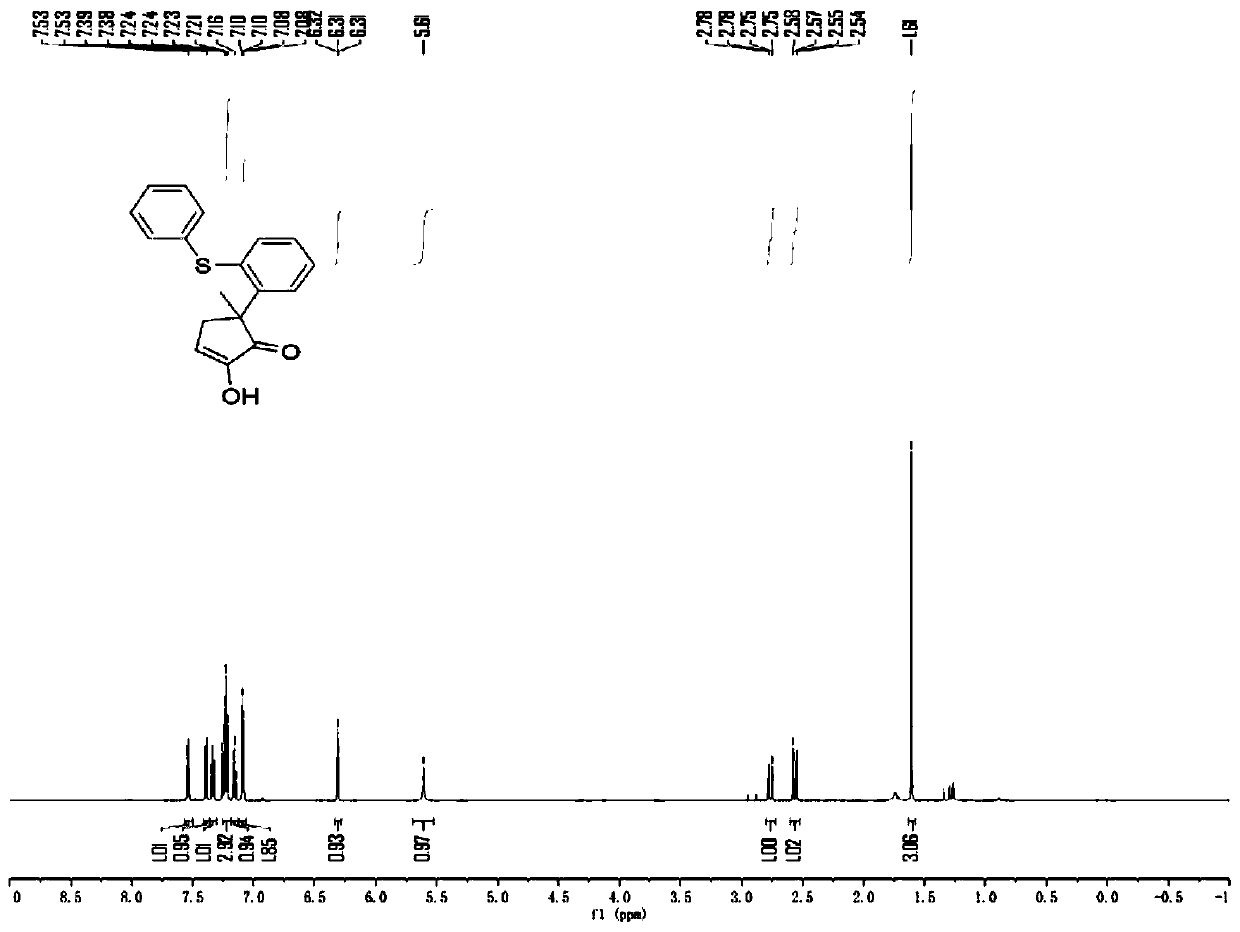
[0046]
[0047] Into a 20mL test tube, add 4-bromophenylthiophene sulfoxide (30mg, 0.1mmol) and 1.2-cyclohexanedione (17.1mg, 0.15mmol) successively at room temperature, and add 0.8ml of dichloroethane at the same time, put In a -10°C low-temperature reactor, wait for the reaction system to mix evenly and the reaction body will be -10°C, add trifluoroacetic anhydride (21mg, 0.15mmol), react at a low temperature of -10°C for 4h, and then raise the temperature of the reaction mixture to room temperature (typically 25°C) and stirred at this temperature for 1 min. After completion, the reaction system in the test tube was transferred to a 10ml eggplant-shaped bottle without any post-processing operation. Using a Heidolph rotary evaporator, the rotation speed was 80-100rpm, the temperature was 38°C, and the vacuum degree was 0.1Mpa. The residue was then subjected to column chromatography using 200-mesh column chromatography silica gel, and the developer was petroleum ether: ethy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com