A preparation method for electrochemical oxidation of chlorinated alkyne compounds in a diaphragmless electrolyzer
A technology for chemical oxidation and chlorination of alkynes, which is applied in the direction of electrolytic components, electrolytic process, electrolytic organic production, etc., can solve the problems of expensive, low atomic efficiency, excessive strong oxidant, etc., and achieve the elimination of metal residues, high economic applicability, Broad substrate range effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]
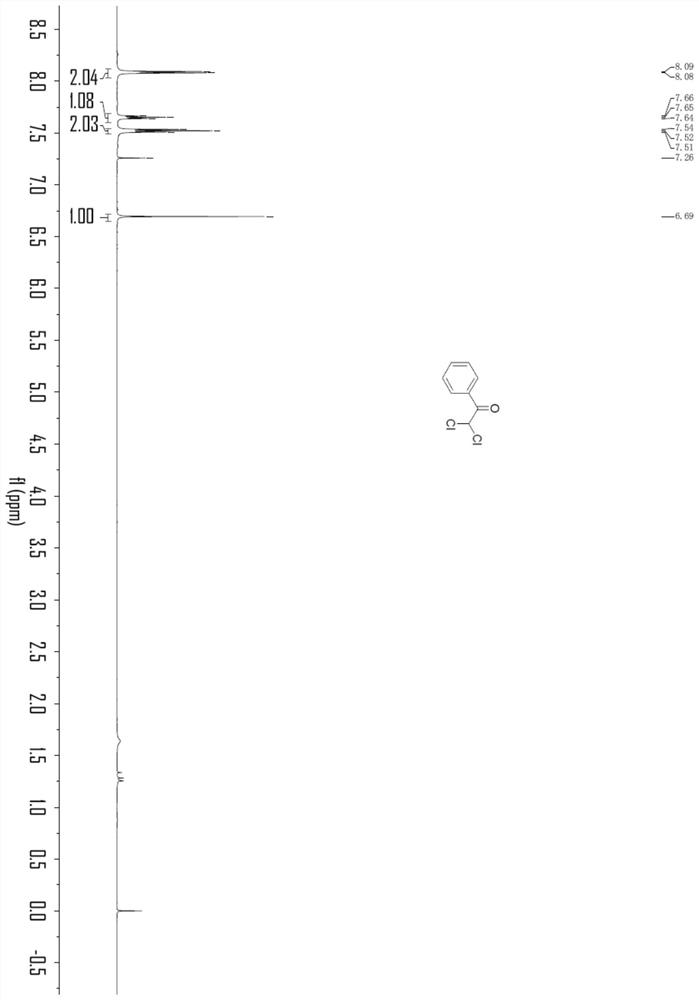
[0034] In an oven-dried three-necked flask (25 mL) equipped with a stir bar, combine phenylacetylene (0.3 mmol), LiClO 4 (1.0 equivalent), nBu 4 NI (0.8 equiv.), H 2 O (2.0 equiv), chloroform (33 equiv) and CH 3 CN (9.8 mL) was mixed and added. The flask was equipped with platinum electrodes (1.0 cm x 1.0 cm x 0.2 mm) as anode and cathode. The reaction mixture was electrolyzed at room temperature with constant current stirring at 10 mA for 6 h. After completion, without post-processing operation, transfer the reaction system in the test tube to a 25mL eggplant-shaped bottle, use a Heidolph rotary evaporator, the rotation speed is 80-100rpm, the temperature is 38°C, and the vacuum degree is 0.1Mpa. The residue was then subjected to column chromatography using 200-mesh column chromatography silica gel, the developing solvent was petroleum ether:ethyl acetate=20:1, and the target compound (48.8 mg, the yield was 86%, and the purity was 98 by HPLC analysis) was iso...
Embodiment 2
[0037]
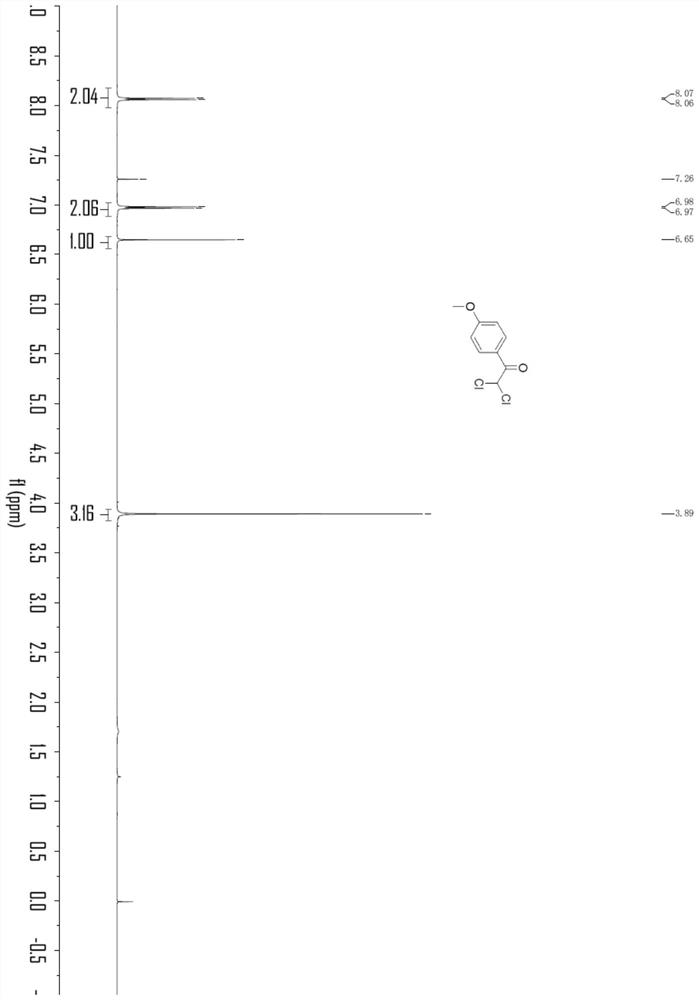
[0038] In an oven-dried three-necked flask (25 mL) equipped with a stir bar, p-methoxyphenylacetylene (0.3 mmol), LiClO 4 (1.0 equivalent), nBu 4 NI (0.8 equiv.), H 2 O (2.0 equiv), chloroform (33 equiv) and CH 3 CN (9.8 mL) was mixed and added. The flask was equipped with platinum electrodes (1.0 cm x 1.0 cm x 0.2 mm) as anode and cathode. The reaction mixture was electrolyzed at room temperature with constant current stirring at 10 mA for 6 h. After completion, without post-processing operation, transfer the reaction system in the test tube to a 25mL eggplant-shaped bottle, use a Heidolph rotary evaporator, the rotation speed is 80-100rpm, the temperature is 38°C, and the vacuum degree is 0.1Mpa. The residue was then subjected to column chromatography using 200-mesh column chromatography silica gel, and the developing solvent was petroleum ether:ethyl acetate=20:1, and the target compound (51.9 mg, yield 79%, purity 98% by HPLC analysis) was isolated. %, it ...
Embodiment 3
[0041]
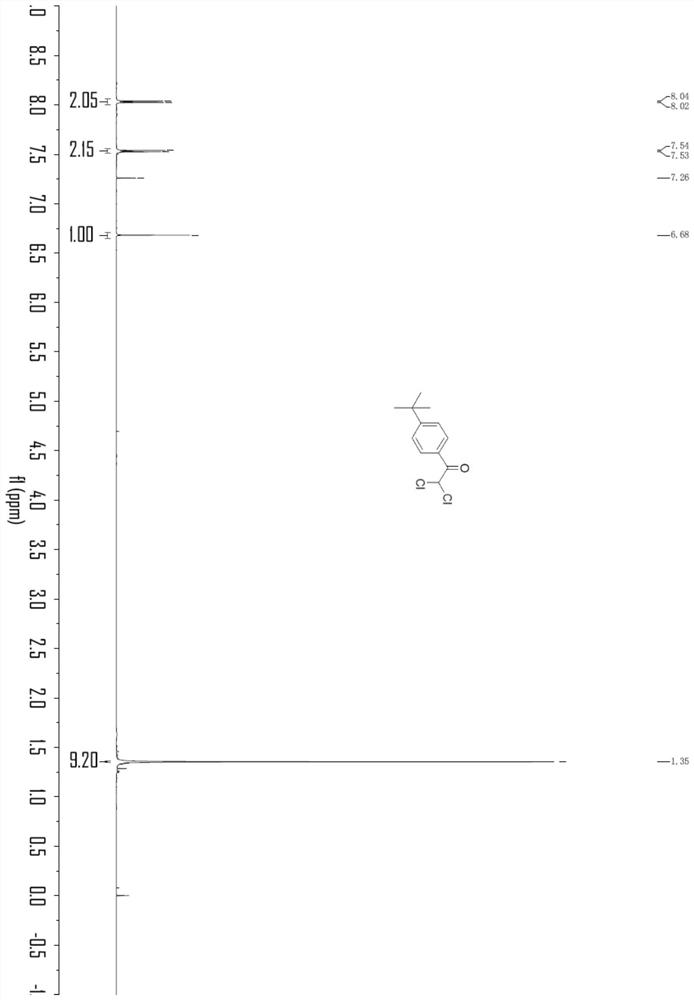
[0042] In an oven-dried three-necked flask (25 mL) equipped with a stir bar, p-tert-butylphenylacetylene (0.3 mmol), LiClO 4 (1.0 equivalent), nBu 4 NI (0.8 equiv.), H 2 O (2.0 equiv), chloroform (33 equiv) and CH 3 CN (9.8 mL) was mixed and added. The flask was equipped with platinum electrodes (1.0 cm x 1.0 cm x 0.2 mm) as anode and cathode. The reaction mixture was electrolyzed at room temperature with constant current stirring at 10 mA for 6 h. After completion, without post-processing operation, transfer the reaction system in the test tube to a 25mL eggplant-shaped bottle, use a Heidolph rotary evaporator, the rotation speed is 80-100rpm, the temperature is 38°C, and the vacuum degree is 0.1Mpa. The residue was then subjected to column chromatography using 200-mesh column chromatography silica gel, the developing solvent was petroleum ether:ethyl acetate=20:1, and the target compound (55.1 mg, the yield was 75%, and the purity was 98 by HPLC analysis) was i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com