A method for the synthesis of α,α-dibromoketones by electrochemical oxidation of dihalogenated alkynes without diaphragm
A compound and electrochemical technology, which is applied in the field of diaphragmless electrochemical oxidation of dihalogenated alkynes to synthesize α,α-dibromoketones, which can solve the problems of inactive halide ions, low efficiency of halogen atoms, complex reactions, etc. , to achieve the effect of eliminating metal residues, high economic applicability, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
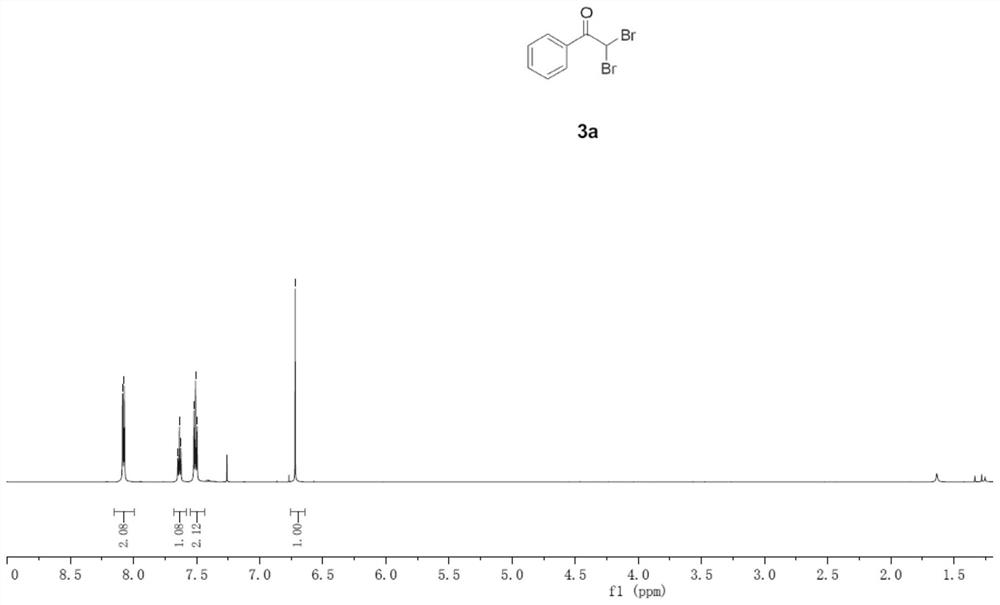
Embodiment 1
[0033] (1) In an oven-dried three-necked flask (25 mL) equipped with a stirring bar, phenylacetylene (0.3 mmol), LiClO4 (1.0 equivalent), nBu4NI (0.8 equivalent), H2O (2.0 equivalent), dibromomethane (33 equiv) and CH3CN (9.8 mL) were mixed and added to give a reaction mixture.
[0034] (2) Platinum electrodes (1.0 cm×1.0 cm×0.2 mm) were installed in the flask as anode and cathode. The reaction mixture was stirred and electrolyzed at room temperature at a constant current of 10 mA for 6 h. After completion, the reaction system in the test tube was transferred to a 25mL eggplant-shaped bottle without any post-processing operation. Using a Heidolph rotary evaporator, the rotation speed was 80-100rpm, the temperature was 38°C, the vacuum degree was 0.1Mpa, and the reaction system was treated for 3 minutes. The residue was subjected to column chromatography using 200-mesh column chromatography silica gel, and the developer was petroleum ether: ethyl acetate = 20:1, and 61.7 mg of...
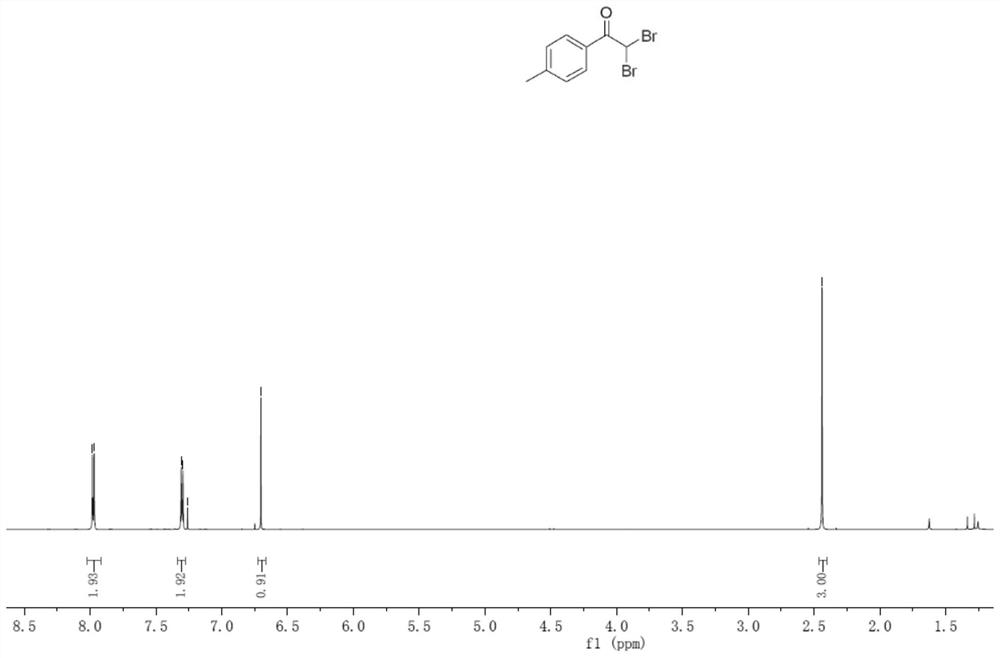
Embodiment 2
[0039] (1) In an oven-dried three-necked flask (25 mL) equipped with a stirring bar, p-tolylphenylene vinylene (0.3 mmol), LiClO4 (1.0 equivalent), nBu4NI (0.8 equivalent), H2O (2.0 equivalent) , dibromomethane (33 equiv) and CH3CN (9.8 mL) were combined and added to give a reaction mixture.
[0040] (2) The flask was equipped with platinum electrodes (1.0 cm x 1.0 cm x 0.2 mm) as anode and cathode. The reaction mixture was stirred and electrolyzed at room temperature at a constant current of 10 mA for 6 h. After completion, the reaction system in the test tube was transferred to a 25mL eggplant-shaped bottle without any post-processing operation. Using a Heidolph rotary evaporator, the rotation speed was 80-100rpm, the temperature was 38°C, the vacuum degree was 0.1Mpa, and the reaction system was treated for 3min. The residue was subjected to column chromatography using 200-mesh column chromatography silica gel, and the developer was petroleum ether: ethyl acetate = 20:1, a...
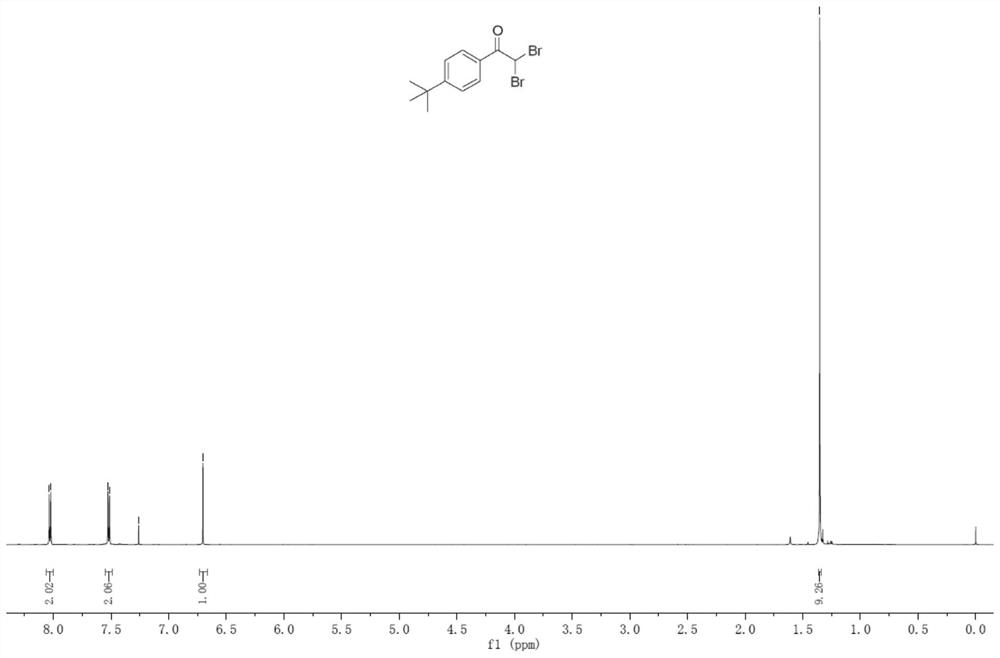
Embodiment 3
[0045] (1) In an oven-dried three-neck flask (25 mL) equipped with a stirring bar, mix p-tert-butylphenylacetylene (0.3 mmol), LiClO4 (1.0 equivalent), nBu4NI (0.8 equivalent), H2O (2.0 eq), dibromomethane (33 eq), and CH3CN (9.8 mL) were mixed and added to give a reaction mixture.
[0046] (2) The flask was equipped with platinum electrodes (1.0 cm x 1.0 cm x 0.2 mm) as anode and cathode. The reaction mixture was stirred and electrolyzed at room temperature at a constant current of 10 mA for 6 h. After completion, the reaction system in the test tube was transferred to a 25mL eggplant-shaped bottle without any post-processing operation. Using a Heidolph rotary evaporator, the rotation speed was 80-100rpm, the temperature was 38°C, the vacuum degree was 0.1Mpa, and the reaction system was treated for 3min. The residue was subjected to column chromatography using 200-mesh column chromatography silica gel, and the developing solvent was petroleum ether: ethyl acetate = 20:1, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com