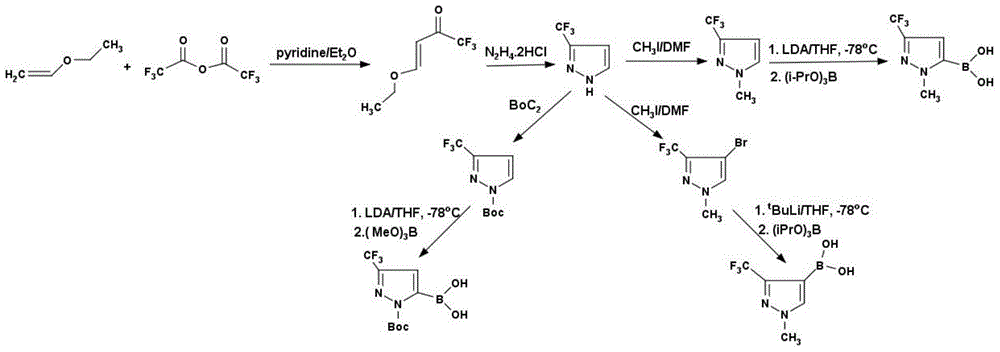
Method for preparing 3-trifluoromethyl pyrrole boric acid
A technology of trifluoromethylpyrrole boronic acid and trifluoromethylpyrrole, which is applied in the field of organic chemical synthesis, and achieves the effects of high yield, good purity, and simple and easy preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation of 4-ethoxy-3-ene trifluoroacetyl ketone:
[0033] Add 1620g of trifluoroacetic anhydride (7.71mol) and 6L of diethyl ether into a 12L reaction flask, cool to 0°C, slowly add 611g of ethylene ethyl ether (8.49mol) and 610g of pyridine (7.71mol) dropwise, complete the addition, and warm to room temperature , stirred for 12 hours, added 2L 1N dilute hydrochloric acid, separated the organic phase, washed with water, washed with saturated sodium chloride, dried with anhydrous magnesium sulfate, concentrated to generate 1200g of 4-ethoxy-3-ene trifluoroacetyl ketone, yield 92 %, 1H NMR (CDCl 3 ): 1.30ppm, triplet (3H); 4.02ppm, quadruplet (2H); 5.82ppm, doublet (1H); 7.82ppm, doublet (1H); 13CNMR (CDCl 3 ): 14.10ppm, 53.37ppm, 97.80ppm, 117.91ppm, 168.10ppm, 180.43ppm.
Embodiment 2
[0035] Preparation of 3-trifluoromethylpyrrole:
[0036] 817g 4-ethoxy-3-ene trifluoroacetylketone (4.86mol) and 510.16g hydrazine dihydrochloride (4.86mol) were dissolved in 2L of absolute ethanol, heated to reflux for 12 hours, cooled to room temperature, and the solvent was removed under reduced pressure, Diluted by adding water, extracted with dichloromethane, separated, dried, concentrated to give 450g of 3-trifluoromethylpyrrole, yield 68%, 1H NMR (CDCl 3 ): 6.71ppm, bimodal (1H); 7.76ppm, bimodal (1H); 13CNMR (CDCl 3 ): 103.82ppm, 123.06ppm, 130.36ppm, 142.70ppm.
Embodiment 3
[0038] Preparation of N-methyl-3-trifluoromethylpyrrole:
[0039] In a 5L reaction flask, dissolve 450g of 3-trifluoromethylpyrrole (3.29mol) in 2L of dimethylformamide, add 682.61g of potassium carbonate (4.94mol), cool to 0°C, and slowly add 701.03g of iodine dropwise Methane (4.94mol), raised to room temperature, stirred for 12 hours, quenched with water, extracted with ether, separated, dried, concentrated, and distilled under reduced pressure to obtain 385.49g of N-methyl-3-trifluoromethylpyrrole, Yield 78%, 1H NMR (CDCl 3 ): 4.85ppm, unimodal (3H); 6.50ppm, bimodal (1H); 7.35ppm, bimodal (1H); 13CNMR (CDCl 3 ): 39.19ppm, 104.39ppm, 122.78ppm, 131.46ppm, 142.31ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com