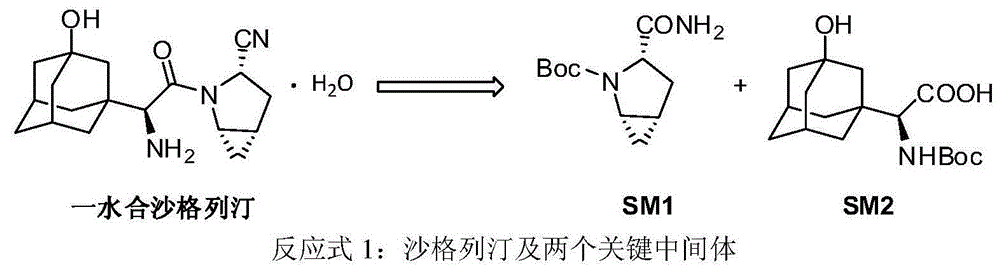
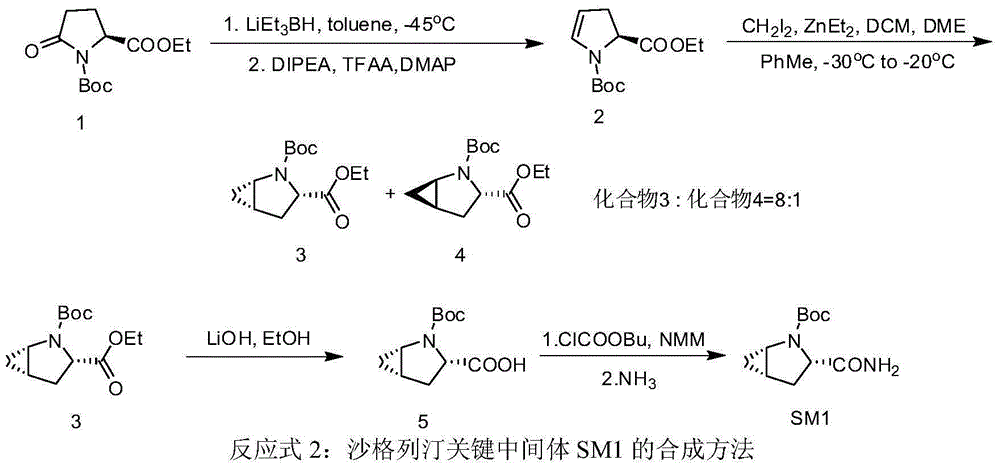
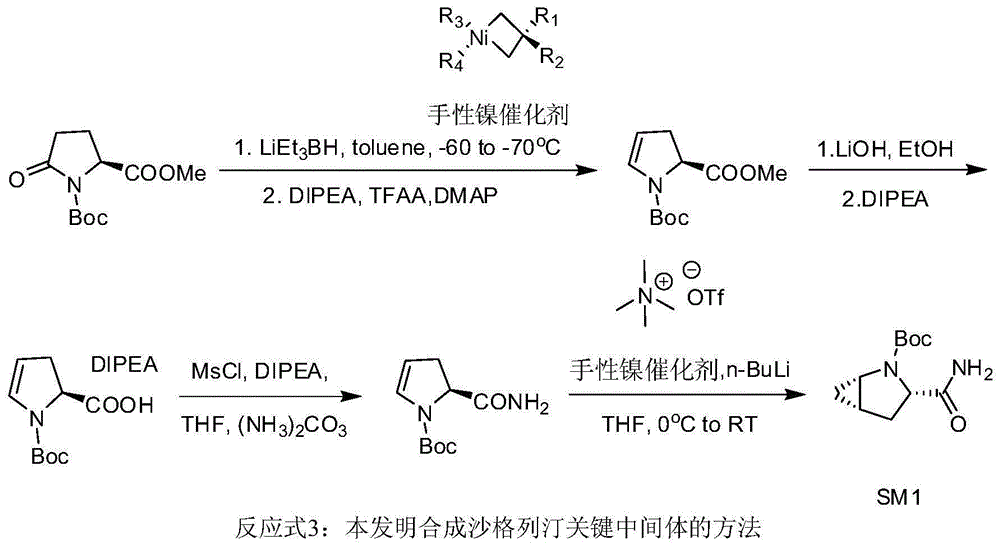
Preparation method of key intermediate of saxagliptin
The technology of tert-butoxycarbonyl and dihydrogen, which is applied in the field of preparation of compound intermediates, can solve the problems of environmental pollution, low cyclopropanation conversion rate and high cost, avoid environmental pollution problems, have high reaction selectivity and reduce production. cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Preparation of (S)-1-N-tert-butoxycarbonyl-2,3-dihydro-2-pyrrolecarboxylic acid methyl ester
[0033] In a 500 mL three-neck flask, under nitrogen protection, Boc-methyl pyroglutamate (21.8 g) and toluene (144 mL) were added. Stir to dissolve, and cool the system temperature to -60°C ~ -70°C. Keeping the temperature at -60°C to -70°C, a solution of lithium triethylborohydride in tetrahydrofuran (1 mol / L, 91.6 mL) was slowly added dropwise, and stirring was continued for 3 hours after the dropwise addition. Add DIPEA, DMAP, and TFAA to the reaction liquid in sequence, and control the temperature not to exceed -60°C. Naturally warmed to room temperature, stirring was continued for 3 hours. Cool to 0-5°C, add water (200 mL), and stir for 20 minutes. After standing still for liquid separation, the organic phase was washed successively with 3 mol / L dilute hydrochloric acid (200 mL), saturated sodium bicarbonate solution (200 mL), and saturated saline solution. ...
Embodiment 2
[0034] Example 2: Preparation of (S)-1-N-tert-butoxycarbonyl-2,3-dihydro-2-pyrrolecarboxylic acid N,N-diisopropylethylamine salt
[0035] In a 500 mL three-necked flask, (S)-1-N-tert-butoxycarbonyl-2,3-dihydro-2-pyrrolecarboxylic acid methyl ester (20.3 g) and methanol (100 mL) were added. The temperature of the reaction solution was cooled to 0-5°C, and aqueous lithium hydroxide solution (0.1 g / mL, 70.7 mL) was slowly added dropwise. After the dropwise addition was completed, the mixture was raised to room temperature and stirred for 2 hours. After the reaction solution was concentrated to half volume at 40°C, water (100 mL) and methyl tert-butyl ether (100 mL) were added, and stirring was continued for 10 minutes. After standing still for liquid separation, the aqueous phase was collected, and methyl tert-butyl ether (100 mL) was added. Cool the mixture to 5-10°C, and adjust the pH to 2.3 with a mixed solution (1:4) of 85% phosphoric acid-water (1:4). Keep the temperature...
Embodiment 3
[0036]Example 3: Preparation of (S)-1-N-tert-butoxycarbonyl-2,3-dihydro-2-pyrrole carboxamide
[0037] In a 500mL three-necked flask, add (S)-1-N-tert-butoxycarbonyl-2,3-dihydro-2-pyrrolecarboxylic acid N,N-diisopropylethylamine salt (26.5g), tetrahydrofuran (150mL ), stir to dissolve. The temperature of the system was lowered to -20°C, methanesulfonyl chloride (13.2g) was slowly added dropwise, and the temperature was controlled not to exceed 0°C, after the dropwise addition, DIPEA (36mL) was added. After stirring for 3 hours, the temperature was controlled not to exceed 0°C. Ammonium carbonate (13.0 g) was added and stirring was continued overnight at room temperature. The reaction was filtered, and the filter cake was washed with an appropriate amount of tetrahydrofuran, and the filtrate was collected and concentrated to dryness at 40°C. The concentrate was dissolved with dichloromethane (150mL), washed with water (150mL), allowed to stand for liquid separation, and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com