Method for synthesizing optically active benzo carboxylic acid ester compound through asymmetric addition reaction
An ester compound, addition reaction technology, applied in organic chemistry methods, organic chemistry and other directions, can solve problems to be explored, and achieve the effects of good enantioselectivity, simple post-processing and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022]
[0023]
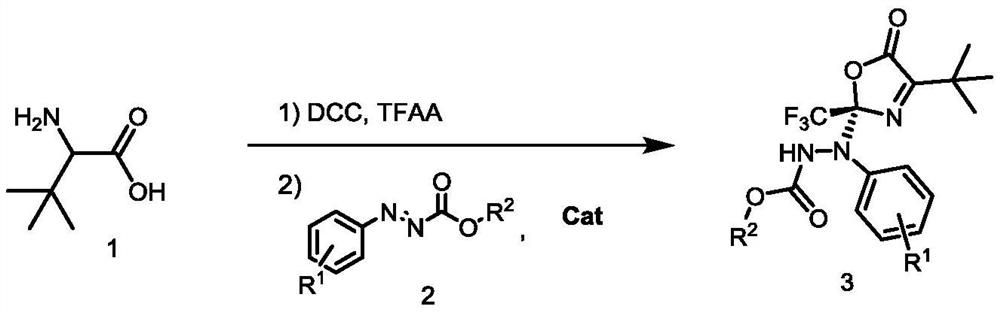
[0024] a tert-leucine 1, trifluoroacetic anhydride, dicyclohexylcarbodiimide, and 1.0 mL of anhydrous solvent were stirred well at room temperature for several hours. Then the catalyst (0.01 mmol, 10 mol%) and azocarboxylate 2a (0.10 mmol) were slowly added to the reaction system and stirred for 12 hours. b The reaction time of the first step. c Isolated yield. d The ee value is obtained by chiral column HPLC chiral analysis.
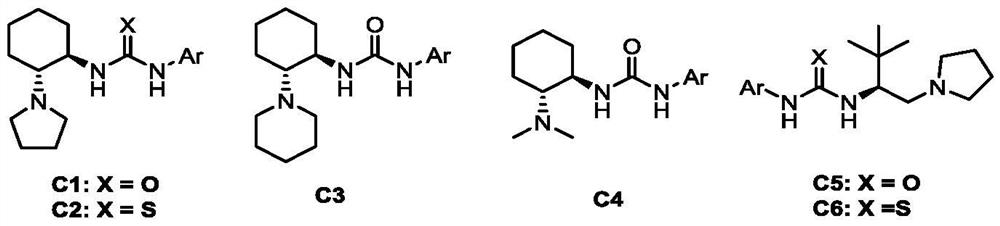
[0025] In the screening process of the reaction conditions, the influence of different catalysts on the reaction (entries 1-6) was first investigated, and finally the catalyst C3 was determined to be the best catalyst, and then the equivalent ratio of dicyclohexylcarbodiimide was investigated. Influence (entries 6-9), finally determined that the equivalent of dicyclohexylcarbodiimide is 8 times equivalent. Subsequently, the influence of the equivalent ratio of tert-leucine on the reaction was examined (entries 9-10), and it w...
Embodiment 2
[0029]
[0030] At room temperature, 1 mL of mesitylene was added to a 10 mL round-bottomed flask after anhydrous treatment, and tert-leucine 1 (26.2 mg, 0.20 mmol, 2 eq), trifluoroacetic anhydride (50.4 mg, 0.24 mmol, 2.4 eq ) and dicyclohexylcarbodiimide (165.0mg, 0.8mmol, 8eq) were added sequentially and stirred for 8 hours, then azocarboxylate 2b (23.1mg, 0.10mmol) and catalyst C3 (4.4mg) were added slowly In the solvent, stir at 25°C for 12h. TLC spot plate tracked until raw material 2b disappeared, filtered to remove insoluble matter, filter residue was washed with dichloromethane, and directly flash silica gel column chromatography (eluent sherwood oil / ethyl acetate=1 / 10-1 / 5 after decompression solvent removal) ) separation and purification to obtain light yellow oily product 6b, the yield is 82%; HPLC (CHIRALPAK IF, n-hexane / isopropanol=90 / 10, flow rate 1.0mL / min, λ=220nm)t R (1)=6.297min,t R (2)=6.803min, 89%ee; (c 1.0, CHCl 3 ); 1 H NMR (600MHz, CDCl 3 )δ7....
Embodiment 3
[0032]
[0033] At room temperature, 1 mL of mesitylene was added to a 10 mL round-bottomed flask after anhydrous treatment, and tert-leucine 1 (26.2 mg, 0.20 mmol, 2 eq), trifluoroacetic anhydride (50.4 mg, 0.24 mmol, 2.4 eq ) and dicyclohexylcarbodiimide (165.0mg, 0.8mmol, 8eq) were added sequentially and stirred for 8 hours, then azocarboxylate 2c (22.4mg, 0.10mmol) and catalyst C3 (4.4mg) were added slowly In the solvent, stir at 25°C for 12h. TLC point plate tracked until the raw material 2c disappeared, filtered to remove insoluble matter, and the filter residue was washed with dichloromethane. After removing the solvent under reduced pressure, direct flash silica gel column chromatography (eluent was petroleum ether / ethyl acetate=1 / 10-1 / 5) Separation and purification to obtain colorless oily product 3c with a yield of 86%; HPLC (NuAnalytical-FLMNZ 2 , n-hexane / isopropanol=94 / 6, flow rate 1.0mL / min, λ=220nm)t R (1) = 6.447min, t R (2)=7.957min, 92%ee; (c 1.0, CH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com