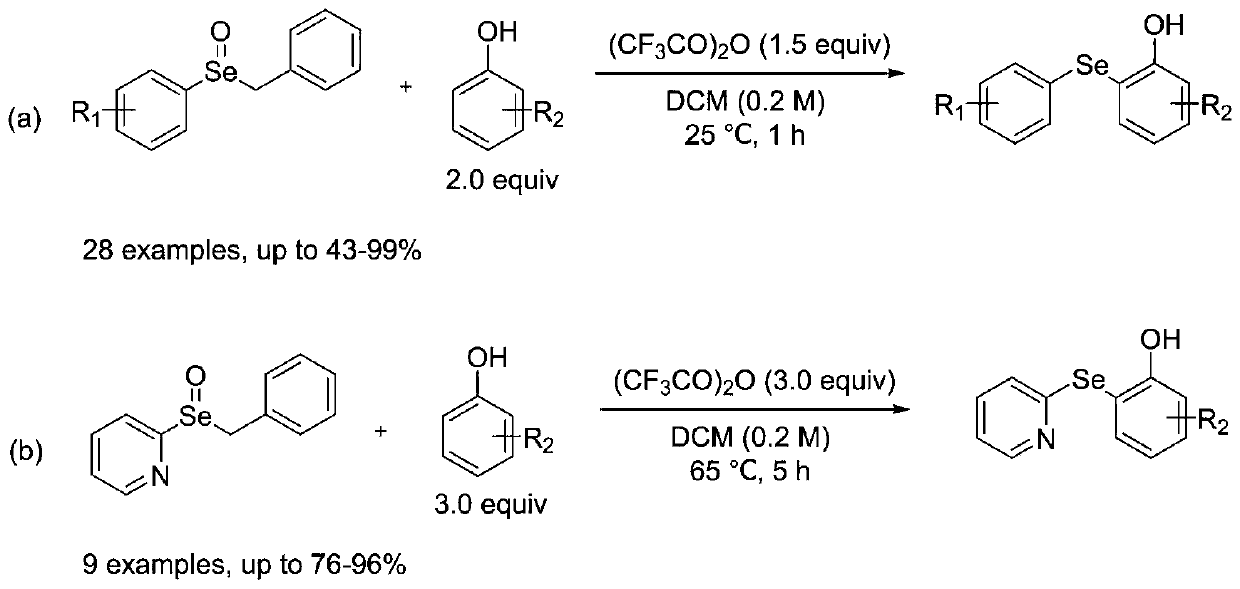
Synthetic method for diaryl selenide
A synthesis method and technology for selenide, applied in the direction of organic chemistry and the like, can solve the problems of high cost of removing transition metal residues, expensive transition metal catalysts, toxicity, etc., and achieve excellent functional group compatibility, mild reaction conditions, and environment-friendly effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
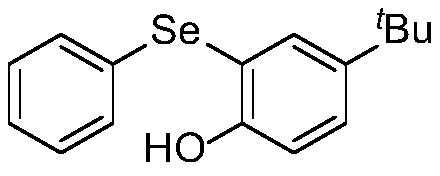
[0019] Embodiment 1: The structural formula of diaryl selenide (4-(tert-butyl)-2-(phenylselenyl)phenol) in this embodiment is shown in the following formula
[0020]
[0021] Denoted as compound 1;
[0022] The synthetic method of 4-(tert-butyl)-2-(phenylselenyl)phenol, concrete steps are as follows:
[0023] (1) In a nitrogen atmosphere, dissolve arylbenzylselenide oxide (phenylbenzylselenide oxide) and phenolic compound (p-tert-butylphenol) in anhydrous dichloromethane, and activate by adding Agent trifluoroacetic anhydride obtains arylbenzyl selenide oxide reaction system; Wherein the mol ratio of aryl benzyl selenide oxide, phenolic compound and trifluoroacetic anhydride is 1:2.0:1.5, aryl benzyl selenide oxide The mass volume ratio g:mL with anhydrous dichloromethane is 1:20.0;
[0024] (2) Seal the arylbenzylselenide oxide reaction system in step (1) in a nitrogen atmosphere, react for 1 h at room temperature under stirring conditions, add saturated sodium bicarbona...
Embodiment 2
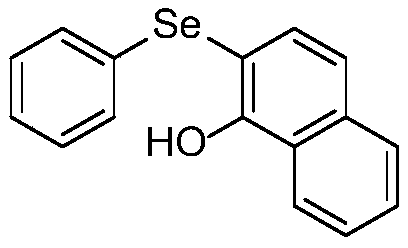
[0030] Embodiment 2: The structural formula of diaryl selenide (2-(phenylselenyl)-1-naphthol) of this embodiment is shown in the following formula
[0031]
[0032] Denoted as compound 2;
[0033] The synthetic method of 2-(phenylselenyl)-1-naphthol, concrete steps are as follows:
[0034] (1) In a nitrogen atmosphere, dissolve arylbenzylselenide oxide (phenylbenzylselenide oxide) and phenolic compound (1-naphthol) in anhydrous dichloromethane, add activator at room temperature Trifluoroacetic anhydride obtains aryl benzyl selenide oxide reaction system; wherein the mol ratio of aryl benzyl selenide oxide, phenolic compound and trifluoroacetic anhydride is 1:2.0:1.5, aryl benzyl selenide oxide and anhydrous The mass volume ratio of dichloromethane g:mL is 1:20.0;
[0035] (2) Seal the arylbenzylselenide oxide reaction system in step (1) in a nitrogen atmosphere, react for 1 h at room temperature under stirring conditions, add saturated sodium bicarbonate solution to quenc...
Embodiment 3
[0041] Embodiment 3: The structural formula of diaryl selenide (4-(tert-butyl)-2-(naphthalene-1-selenyl)phenol) in this embodiment is shown in the following formula
[0042]
[0043] Denoted as compound 3;
[0044] The synthetic method of 4-(tert-butyl)-2-(naphthalene-1-selenyl)phenol, concrete steps are as follows:
[0045] (1) In a nitrogen atmosphere, arylbenzylselenide oxide (1-naphthylbenzylselenide oxide) and phenolic compound (p-tert-butylphenol) are dissolved in anhydrous dichloromethane, at room temperature, Add activator trifluoroacetic anhydride to obtain arylbenzylselenide oxide reaction system; wherein the molar ratio of arylbenzylselenide oxide, phenolic compound and trifluoroacetic anhydride is 1:2.5:2.0, arylbenzylselenide oxide The mass volume ratio g:mL with anhydrous dichloromethane is 1:25.0;
[0046] (2) Seal the arylbenzylselenide oxide reaction system in step (1) in a nitrogen atmosphere, react for 2 hours at room temperature under stirring conditio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com