Fluorine-containing potential dual-function probe based on chitosan structure and preparation method thereof
A chitosan, dual-function technology is applied in the field of novel fluorine-containing magnetic resonance imaging contrast agents and their preparation, which can solve the problems of fluctuation and inability to form MR images, and achieve the effect of good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
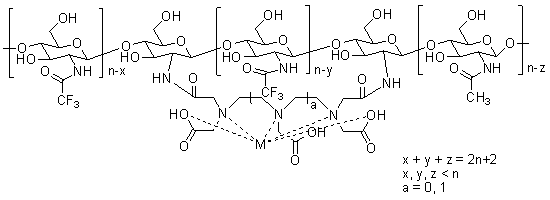
[0027] Example 1: the fluorine-containing magnetic resonance contrast agent (CF 3 -CS 11 -Gd-DTPA-2)
[0028]
[0029] Where x, y, z=3,4,5,6,7,8,9,10.
[0030] Under the protection of nitrogen, 140.2 mg (average molecular weight about 2337, 0.06 mmol) of chitosan oligosaccharide with a degree of polymerization of 11 chained with diethylenetriaminepentaacetic acid (DTPA) and 66.0 mg of 4-dimethylaminopyridine (0.54 mmol) into a 50ml dry round bottom flask, and 1mL of dry dimethylformamide was added to form a suspension, then 113.4mg (0.54mmol) of trifluoroacetic anhydride was added dropwise, stirred at 25°C for 24h after the addition was completed, and 3mL of water was added to quench quench the reaction and add GdCl 3 ·6H 2 O (22.3mg, 0.06mmol) continued stirring at 25°C for 6h. After the reaction was completed, it was concentrated and dehydrated in vacuo, 30 mL of acetone was added, allowed to stand for 1 hour, and filtered to obtain 148.4 mg of the target product, wi...
example 2
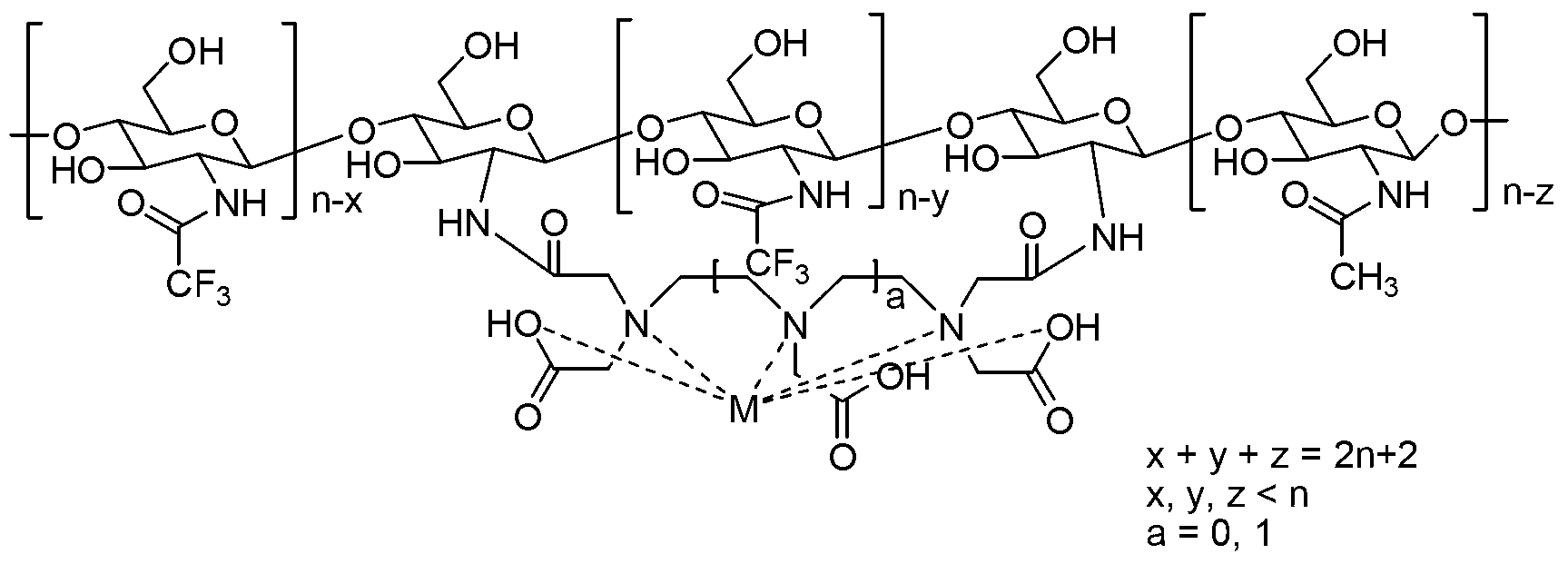
[0032] Example 2: the fluorine-containing magnetic resonance contrast agent (CF 3 -CS 11 -Mn-DTPA-2)
[0033]
[0034] Where x, y, z=3,4,5,6,7,8,9,10.
[0035] Under the protection of nitrogen, 140.2mg (average molecular weight about 2337, 0.06mmol) of chitosan oligosaccharides with a degree of polymerization of 11 chained with diethylenetriaminepentaacetic acid (DTPA) and 54.6mg (0.54mmol) of triethylamine were added Put it into a 50ml dry round bottom flask, add 1mL of dry acetonitrile to form a suspension, then add 113.4mg (0.54mmol) of trifluoroacetic anhydride dropwise, stir at 25°C for 24h after adding, add 3mL of water to quench the reaction, and add MnCl 2 4H 2 O (47.6mg, 0.24mmol) continued to stir at 25°C for 6h. After the reaction was completed, it was concentrated and dehydrated in vacuo, 30 mL of acetone was added, and after standing for 1 h, it was filtered to obtain 130.2 mg of the target product, with a yield of 66.4%.
[0036] The in vitro relaxation rat...
example 3
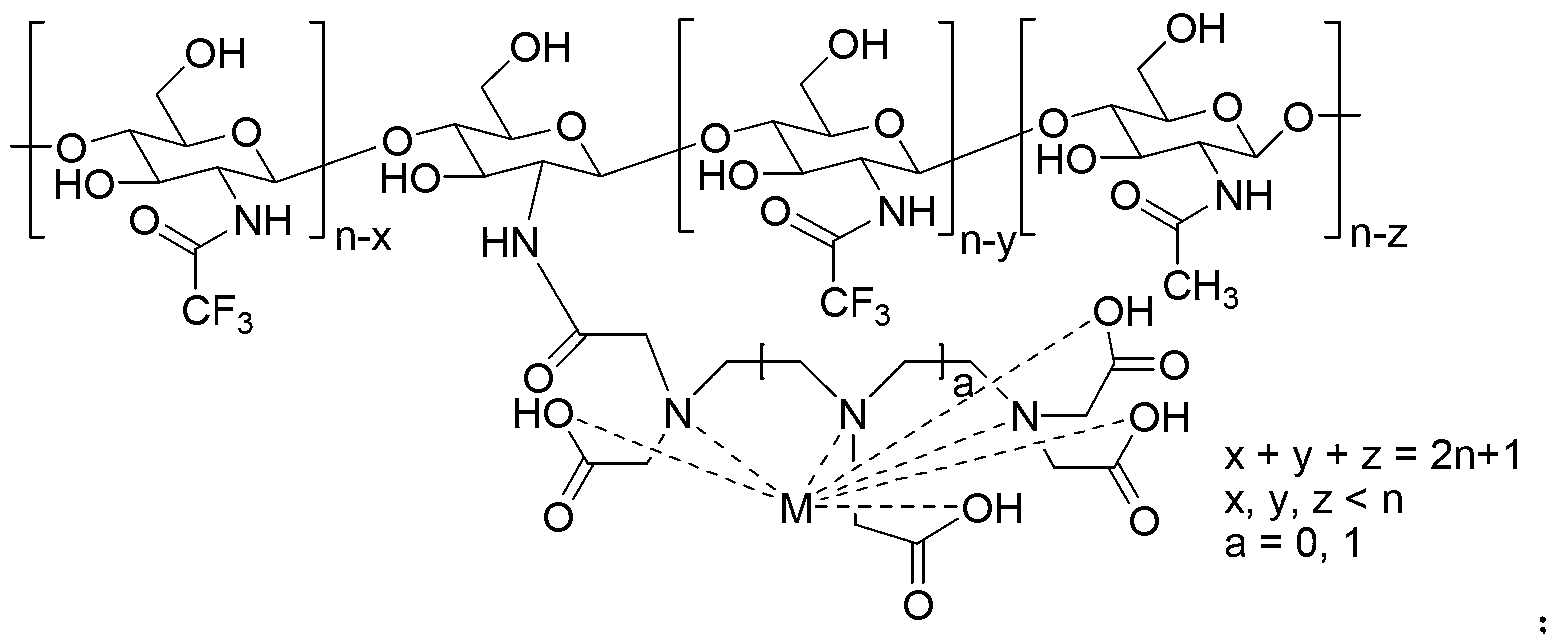
[0037] Example 3: the fluorine-containing magnetic resonance contrast agent (CF 3 -CS 11 -Mn-EDTA-2)
[0038]
[0039] Where x, y, z=3,4,5,6,7,8,9,10.
[0040] Under the protection of nitrogen, 134.2 mg (average molecular weight about 2236, 0.06 mmol) of chitosan oligosaccharides (average molecular weight about 2236, 0.06 mmol) and 54.6 mg (0.54 mmol) of triethylamine were added to the In a 50ml dry round bottom flask, add 1mL dry dimethylacetamide to form a suspension, then add 113.4mg (0.54mmol) trifluoroacetic anhydride dropwise, stir at 25°C for 24h after adding, add 3mL water to quench the reaction, and Add MnCl 2 4H 2 O (11.9mg, 0.06mmol) continued stirring at 25°C for 6h. After the reaction was completed, it was concentrated and dehydrated in vacuo, and 30 mL of absolute ethanol was added. After standing for 1 h, it was filtered to obtain 123.7 mg of the target product, with a yield of 64.1%.
[0041] The in vitro relaxation rate of this product is 8.5L·mmol -...
PUM
| Property | Measurement | Unit |
|---|---|---|
| dispersity | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com