Synthetic method for trifluoromethyl methylation arene
A technology for trifluoromethylated aromatic hydrocarbons and a synthesis method, which is applied in the field of synthesis of trifluoromethylated aromatic hydrocarbons, can solve the problems of high price and difficulty in obtaining, and achieves high universality, low price of raw materials, and high yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
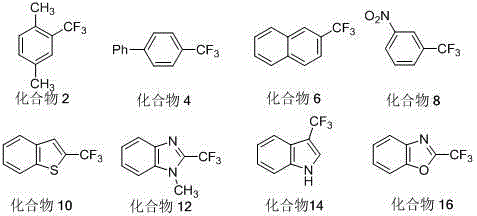
[0017] Embodiment 1: preparation compound 2
[0018] Under the protection of nitrogen, add brominated aromatic compounds successively into a 50 mL pressure-resistant glass reaction sealed tube with a Teflon cock 1 (2.0 mmol), copper powder (20.0 mmol), elemental iodine (20.0 mmol), trifluoroacetic anhydride (20.0 mmol, about 2.82 mL), N,N-dimethylformamide (10.0 mL) and pyridine (5.0 mL ), heated to 180°C after sealing, and reacted for 12 hours. After the reaction, it was naturally cooled to room temperature. The reaction mixture was slowly poured into 100 mL of saturated aqueous sodium bicarbonate solution to quench, extracted with 100 mL of diethyl ether, repeated diethyl ether extraction three times, combined the organic phase, washed once with distilled water and once with saturated brine, dried over anhydrous sodium sulfate, The organic phase was filtered, and the ether solvent was distilled off under reduced pressure by a rotary evaporator to obtain a concentrated or...
Embodiment 2
[0024] Embodiment 2: preparation compound 4
[0025] The preparation method is basically the same as in Example 1, the difference is that the brominated aromatic compound in Example 1 1 Replaced by Brominated Aromatic Compounds 3 , to get the compound 4 Colorless solid 0.320 g (Yield: 72%).
[0026]
[0027] The structural confirmation data of this product are as follows:
[0028] Mass Spectrum: MS (EI): m / z (%) 222 (100), (M + ).
[0029] H NMR spectrum: 1 H NMR (300 MHz, CDCl 3 ) δ (ppm): δ = 7.68 (s, 4H), 7.61-7.58 (m, 2H), 7.49-7.40 (m, 3H).
[0030] NMR fluorine spectrum: 19 F NMR (282 MHz, CDCl 3 ) δ (ppm): δ = -62.3 (s, 3F).
Embodiment 3
[0031] Embodiment 3: preparation compound 6
[0032] The preparation method is basically the same as in Example 1, the difference is that the brominated aromatic compound in Example 1 1 Replaced by Brominated Aromatic Compounds 5 , to get the compound 6 Colorless solid 0.278 g (yield: 71%).
[0033]
[0034] The structural confirmation data of this product are as follows:
[0035] Mass Spectrum: MS (EI): m / z (%) 196 (100), (M + ).
[0036] H NMR spectrum: 1 H NMR (300 MHz, CDCl 3 ) δ (ppm): δ = 8.16 (s, 1H), 7.97-7.90 (m, 3H), 7.67-7.58 (m, 3H).
[0037] NMR fluorine spectrum: 19 F NMR (282 MHz, CDCl 3 ) δ (ppm): δ = -62.2 (s, 3F).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com