Preparation method for alfacalcidol
A technology of alfacalcidol and oxidant, applied in the direction of organic chemistry, etc., can solve the problems of unsuitability for large-scale production, high separation cost, small amount of separated samples, etc., achieve shortened production cycle, high-efficiency preparation method, and avoid low yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
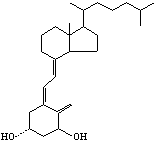
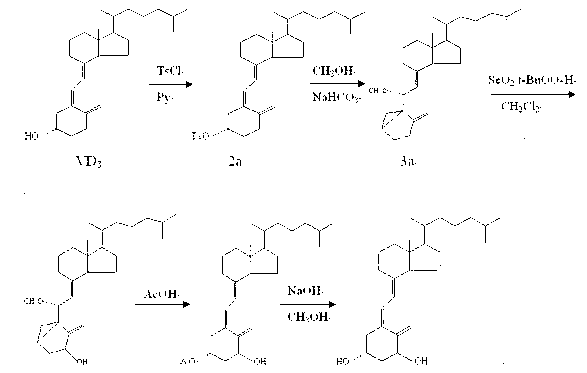
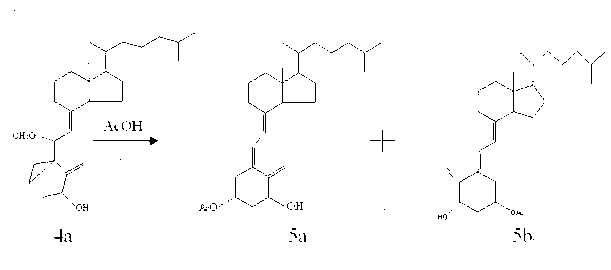
[0016] ⑴Vitamin D 3 Synthesis of p-toluenesulfonate (2a)
[0017] Vitamin D 3 Add 10g and 14g of p-toluenesulfonic acid chloride into the reaction flask, dissolve completely with 100mL of pyridine, mix well and place in the refrigerator (2-4°C) for 48h; add 20g of ice cubes and 100mL of saturated sodium bicarbonate solution, and stir for 15min To decompose excess p-toluenesulfonyl chloride; extract with 1500mL ethyl acetate; the organic layer was washed with 3% dilute hydrochloric acid (500mL×2), saturated sodium bicarbonate solution (500mL×1) and saturated sodium chloride solution (500 mL× 1) Wash, then dehydrate with anhydrous magnesium sulfate, filter; the filtrate is concentrated to dryness under reduced pressure to obtain a light yellow solid that is the crude product VD 3 13.5g p-toluenesulfonate, yield 97.0%, this crude product can be directly used in the next reaction.
[0018] (2) 3,5-cyclovitamin D 3 Synthesis of (3a)
[0019] Add 13.0g of compound 2a, 35g of s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com