Improved preparation method of alfacalcidol
A technology of alfacalcidol and its compounds, which is applied in the field of improved preparation, can solve the problems of difficulty in controlling the conversion rate of cis-isomers, unfavorable scale-up production, and high requirements for equipment investment, so as to avoid the use of preparation liquid phase and facilitate The effect of product purification and labor cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
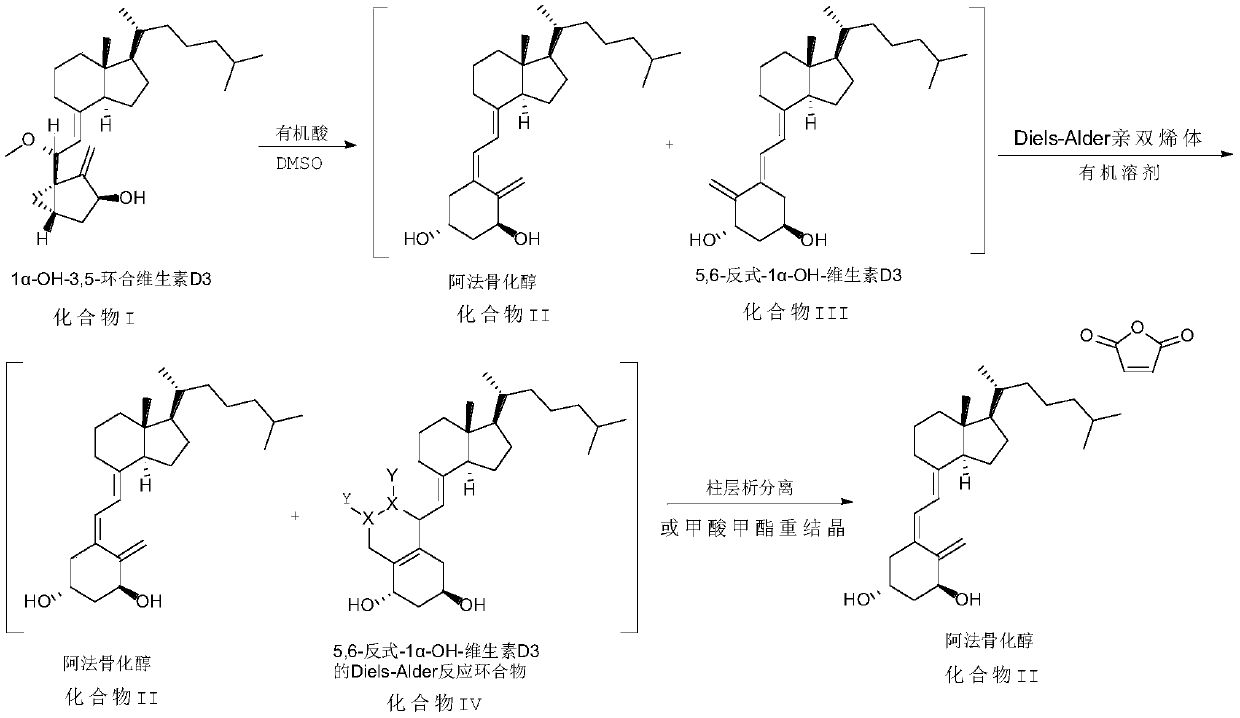
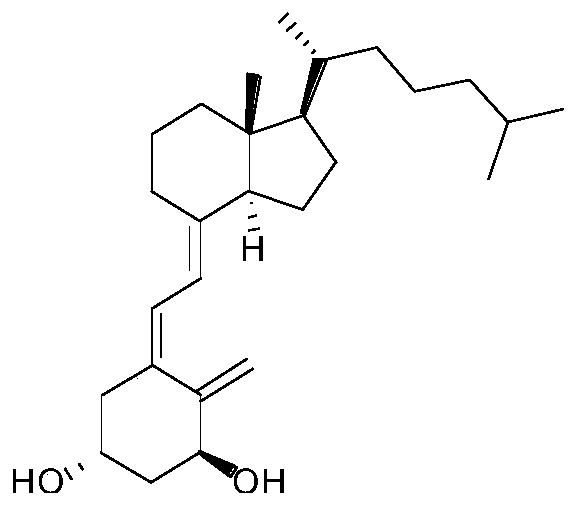
[0030] Add a mixed solvent of 30ml dimethyl sulfoxide and 24ml glacial acetic acid into the reaction flask, add 1αhydroxy-3,5-cyclovitamin D3 (9g, 21.7mmol) at room temperature, and react at 50°C for 1 hour under the protection of nitrogen. The solution was slowly poured into 500ml aqueous sodium bicarbonate solution pre-cooled to 5°C, extracted three times with ether (200ml), the combined organic phases were washed successively with saturated aqueous sodium bicarbonate solution (150ml) and saturated brine (150ml). Dry over magnesium sulfate, filter, and concentrate under reduced pressure to obtain a mixture of alfacalcidol and 5,6-trans-1α-OH-vitamin D3 (trans isomer of alfacalcidol, compound III), with a yield of 100% , used directly in the following reaction.
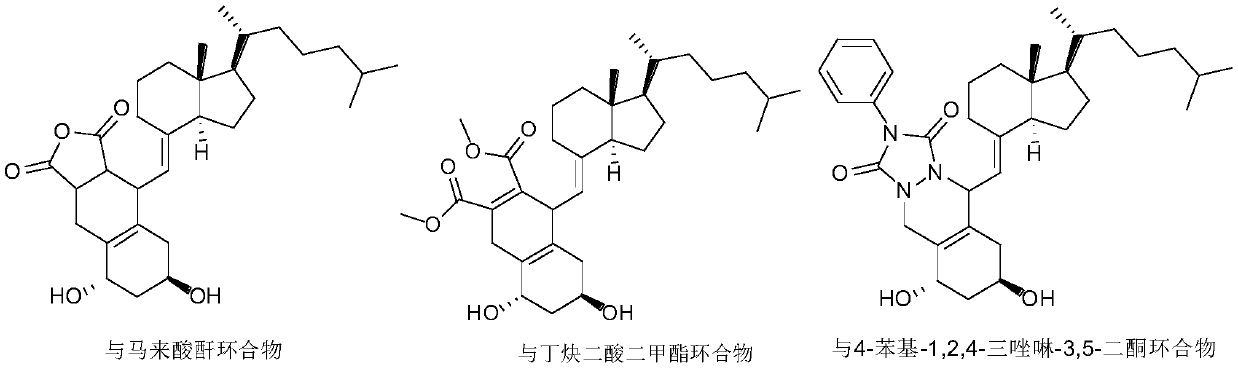
[0031] Add 100ml of ethyl acetate and maleic anhydride (2.12g, 21.7mmol) to the above reaction concentrate, react at 35°C for 24 hours under nitrogen protection, the reaction solution is concentrated under reduced pr...
Embodiment 2
[0034] Add a mixed solvent of 30ml dimethyl sulfoxide and 24ml glacial acetic acid into the reaction flask, add 1αhydroxy-3,5-cyclovitamin D3 (9g, 21.7mmol) at room temperature, and react at 50°C for 1 hour under the protection of nitrogen. The solution was slowly poured into 500ml aqueous sodium bicarbonate solution pre-cooled to 5°C, extracted three times with ether (200ml), the combined organic phases were washed successively with saturated aqueous sodium bicarbonate solution (150ml) and saturated brine (150ml). Dry over magnesium sulfate, filter, and concentrate under reduced pressure to obtain a mixture of alfacalcidol and 5,6-trans-1α-OH-vitamin D3 (trans isomer of alfacalcidol, compound III), with a yield of 100% , used directly in the following reaction.
[0035] Add 100ml of ethyl acetate and dimethyl butyndioate (3.08g, 21.7mmol) to the above reaction concentrate, react at 35°C for 24 hours under nitrogen protection, concentrate the reaction solution under reduced pr...
Embodiment 3
[0038] Add a mixed solvent of 30ml dimethyl sulfoxide and 24ml glacial acetic acid into the reaction flask, add 1αhydroxy-3,5-cyclovitamin D3 (9g, 21.7mmol) at room temperature, and react at 50°C for 1 hour under the protection of nitrogen. The solution was slowly poured into 500ml aqueous sodium bicarbonate solution pre-cooled to 5°C, extracted three times with ether (200ml), the combined organic phases were washed successively with saturated aqueous sodium bicarbonate solution (150ml) and saturated brine (150ml). Dry over magnesium sulfate, filter, and concentrate under reduced pressure to obtain a mixture of alfacalcidol and 5,6-trans-1α-OH-vitamin D3 (trans isomer of alfacalcidol, compound III), with a yield of 100% , used directly in the following reaction.
[0039] Add 100ml of ethyl acetate and 4-phenyl-1,2,4-triazoline-3,5-dione (3.8g, 21.7mmol) to the above reaction concentrate, react at 10°C for 2 hours under nitrogen protection , the reaction solution was concentra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com