Inspection method for alfacalcidol tablet related substances
An inspection method and technology of related substances, which can be used in measuring devices, instruments, scientific instruments, etc., and can solve problems such as lack of inspection methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
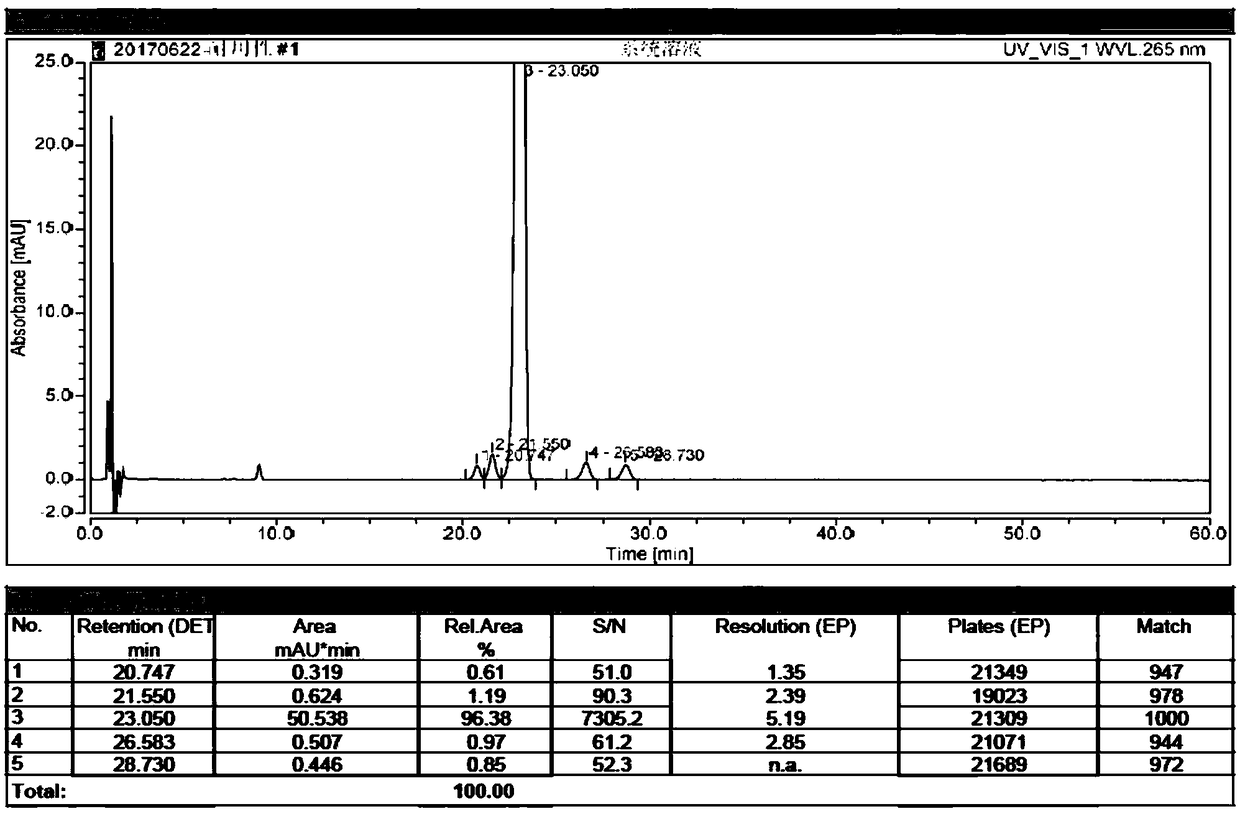
[0075] Embodiment 1: High performance liquid chromatography analysis method of related substances in alfacalcidol tablets, the specification of the tablet is 0.5 μg.
[0076] (1) Take 50 tablets of this product, equivalent to 25μg alfacalcidol, put them in a 50ml Nessler colorimetric tube, add 25ml of water, vortex until the tablets are completely disintegrated, add 10ml of ethyl acetate, shake fully, and let Set to separate layers, take the upper layer solution and concentrate it to dryness under reduced pressure, repeat the above process for a total of three times; add 5ml of methanol / water mixture with a volume ratio of 80:20 to the residue after concentration to dryness, mix well, and use a glass syringe to inject 0.45μm The test solution is obtained by filtering with a polytetrafluoroethylene filter membrane; accurately measure 100 μl of the test solution, place it in a 10ml measuring bottle, dilute to the mark with a methanol / water mixture with a volume ratio of 80:20, an...
Embodiment 2
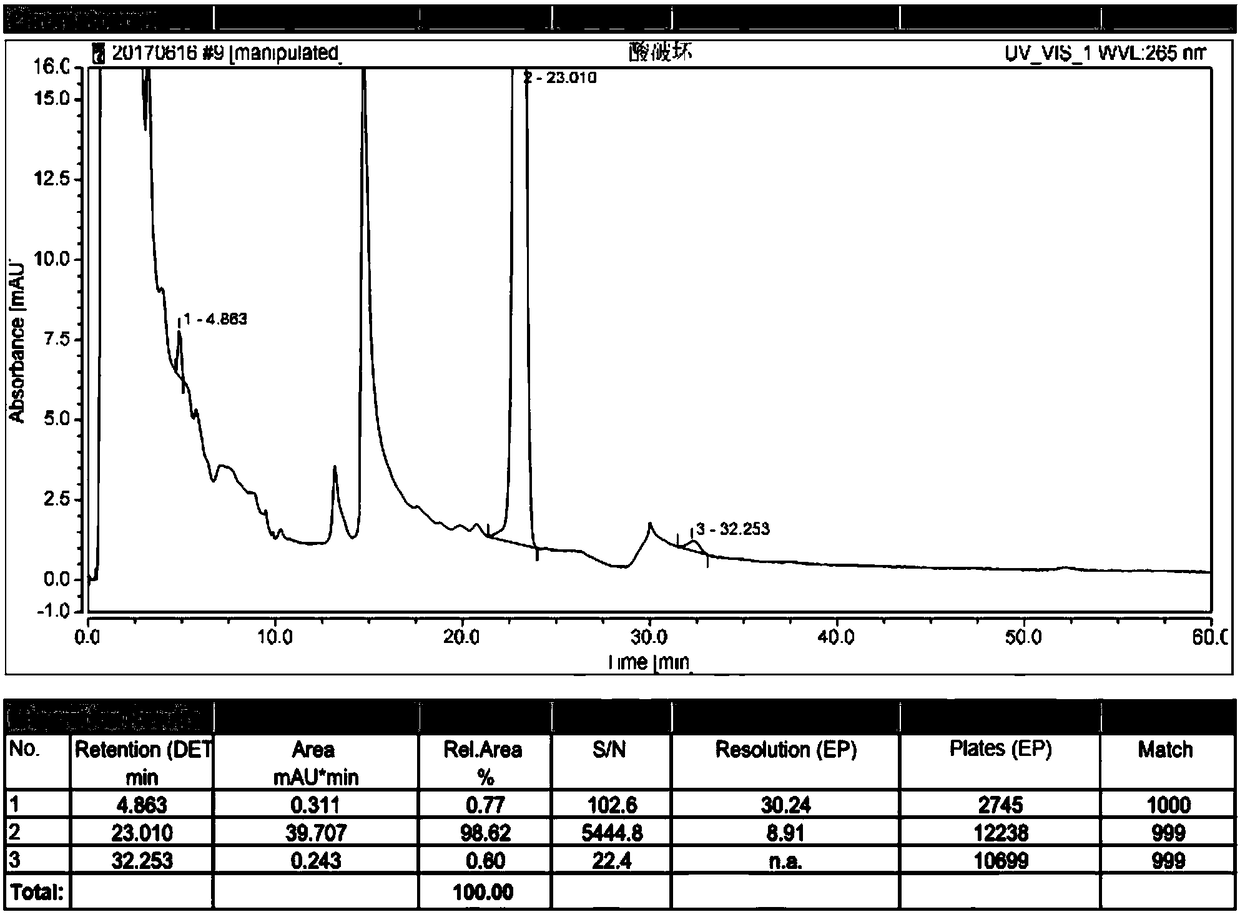
[0088] Embodiment 2: High performance liquid chromatography analysis method of related substances in alfacalcidol tablets, the tablet specification is 0.25 μg.
[0089] (1) Take 50 tablets of this product, equivalent to 25μg alfacalcidol, put them in a 50ml Nessler colorimetric tube, add 25ml of water, vortex until the tablets are completely disintegrated, add 10ml of ethyl acetate, shake fully, and let Set to separate layers, take the upper layer solution and concentrate it to dryness under reduced pressure, repeat the above process for a total of three times; add 2.5ml of methanol / water mixture with a volume ratio of 80:20 to the residue after concentration to dryness, mix well, and inject it through a glass syringe through 0.45 μm polytetrafluoroethylene filter membrane to obtain the test solution; accurately measure 100 μl of the test solution, place it in a 10ml measuring bottle, dilute to the mark with a methanol / water mixture with a volume ratio of 80:20, shake Uniform,...
Embodiment 3
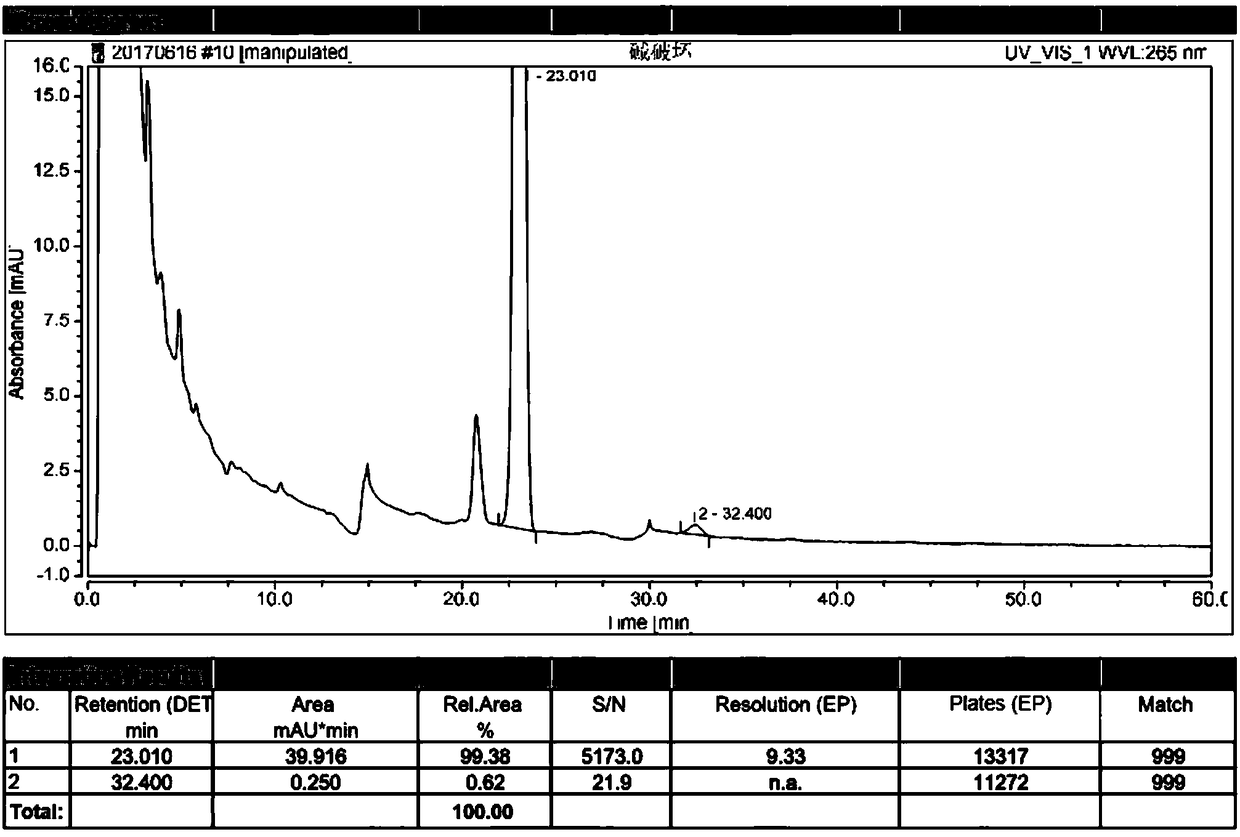
[0101] Example 3 High performance liquid chromatography analysis method for related substances in alfacalcidol tablets, the tablet specification is 0.5 μg.
[0102] (1) Take 50 tablets of this product, equivalent to 25μg alfacalcidol, put them in a 50ml Nessler colorimetric tube, add 25ml of water, vortex until the tablets are completely disintegrated, add 10ml of ethyl acetate, shake fully, and let Set to separate layers, take the upper layer solution and concentrate it to dryness under reduced pressure, repeat the above process for a total of three times; add 5ml of methanol / water mixture with a volume ratio of 80:20 to the residue after concentration to dryness, mix well, and use a glass syringe to inject 0.45μm The test solution is obtained by filtering with a polytetrafluoroethylene filter membrane; accurately measure 100 μl of the test solution, place it in a 10ml measuring bottle, dilute to the mark with a methanol / water mixture with a volume ratio of 80:20, and shake we...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com