A kind of high performance liquid chromatography analysis method of pomalidomide related substance
A technology of high-performance liquid chromatography and analysis method, which is applied in the field of analysis of the purity of chemical drugs, can solve the problems of no relevant literature reports, etc., and achieve the effect of great positive progress, great practical application value, and strong method specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
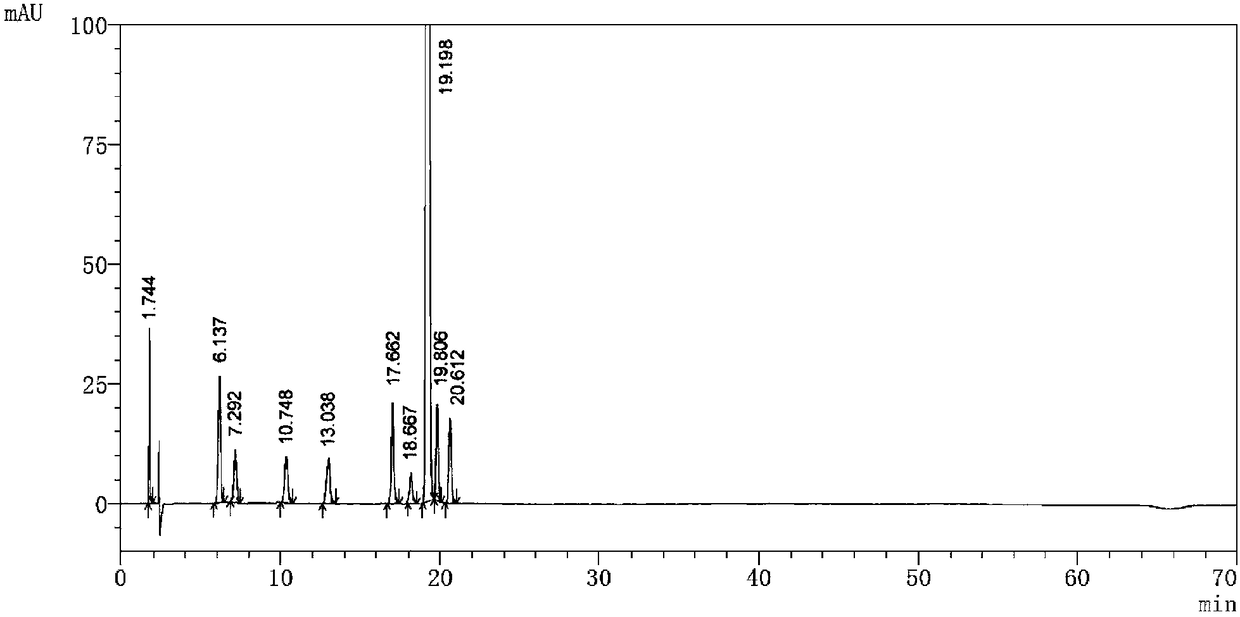
Embodiment 1
[0041] Detection equipment and chromatographic conditions:
[0042] High performance liquid chromatography: LC-10AD pump, SPD-M10A detector
[0043] Chromatographic column: Agilent C18 (150×4.6mm, 5μm); mobile phase A: acetonitrile-water-phosphoric acid solution with a volume ratio of 5:95:0.1; mobile phase B: acetonitrile-water with a volume ratio of 25:75:0.1 - Phosphoric acid solution; detection wavelength: 226nm; flow rate: 1.0ml / min; injection volume: 20μl.
[0044] Experimental steps:
[0045] (1) Sample preparation:
[0046] Take an appropriate amount of pomalidomide and known impurities A-I, add an acetonitrile-water-phosphoric acid solution with a volume ratio of 50:50:0.1 to ultrasonically dissolve and quantitatively dilute the mixed solution to an appropriate concentration, shake well, and use it as a sample solution.
[0047] (2) Gradient elution program setting:
[0048] time (minutes)
Mobile phase A(%)
Mobile phase B(%)
0
100
0
...
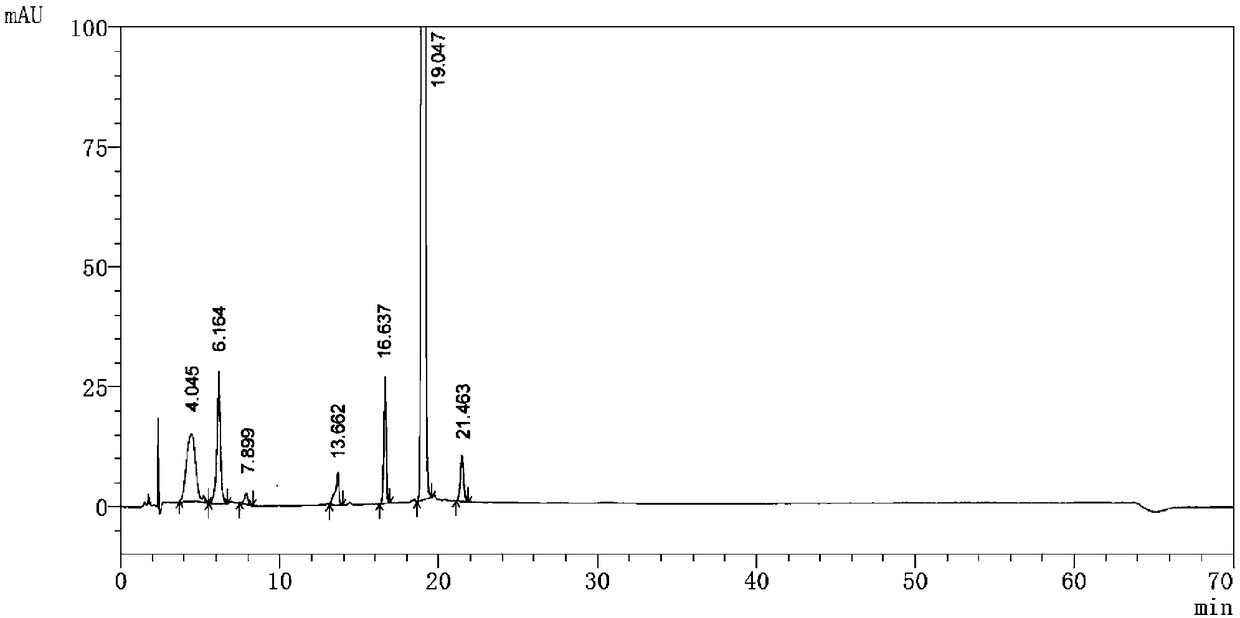
Embodiment 2
[0052] Detection equipment and chromatographic conditions:
[0053] High performance liquid chromatography: LC-10AD pump, SPD-M10A detector
[0054] Chromatographic column: Agilent C18 (150×4.6 mm, 5 μm); mobile phase A: same as Example 1, mobile phase B: same as Example 1; flow rate: 1.0 ml / min; detection wavelength: 226 nm; injection volume: 20 μl.
[0055] (1) Sample preparation:
[0056] Acid destruction: Take the contents of pomalidomide capsules, grind them finely, take an appropriate amount of fine powder (about 20 mg containing pomalidomide), put it in a 100ml measuring bottle, add 4ml of 1mol / L hydrochloric acid solution, and put it in a water bath at 100°C for 1 hour. After 1 hour, take it out and let it cool, add 4ml of 1mol / L sodium hydroxide solution to neutralize it, add an appropriate amount of acetonitrile-water-phosphoric acid solution with a volume ratio of 50:50:0.1 to sonicate to dissolve and dilute to the mark, shake well, filter, and use as acid damage ...
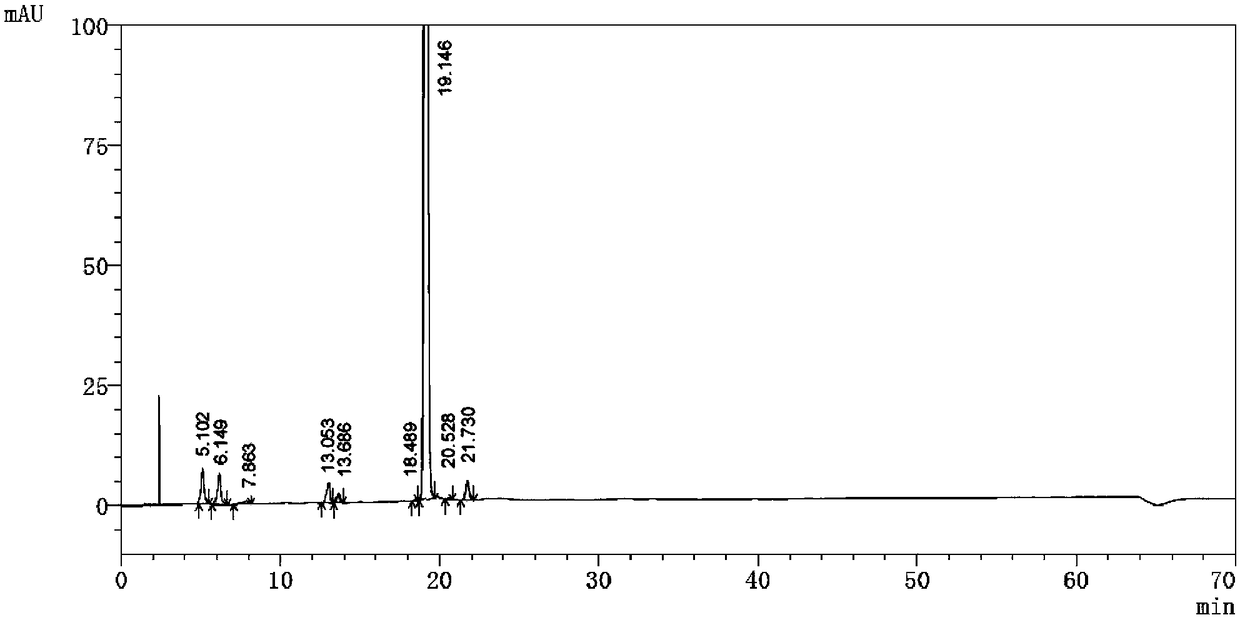
Embodiment 3
[0066] Detection equipment and chromatographic conditions:
[0067] High performance liquid chromatography: UltiMate 3000 pump, UltiMate 3000 UV detector
[0068] Chromatographic column: Agilent C18 (150×4.6 mm, 5 μm); mobile phase A: same as Example 1, mobile phase B: same as Example 1; flow rate: 1.0 ml / min; detection wavelength: 226 nm; injection volume: 20 μl.
[0069] Experimental steps:
[0070] (1) Sample preparation: Take 20 mg of pomalidomide, put it in a 100 ml measuring bottle, dissolve it ultrasonically with an acetonitrile-water-phosphoric acid solution with a volume ratio of 50:50:0.1, dilute to the mark, shake well, and use it as a sample solution.
[0071] (2) Gradient elution program setting:
[0072] time (minutes)
Mobile phase A(%)
Mobile phase B(%)
0
100
0
2
100
0
15
0
100
40
0
100
41
100
0
50
100
0
[0073] (3) Detection: Take the above-mentioned sample solutio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com