Alfacalcidol sustained-release capsule and preparation method thereof
A technology of alfacalcidol and sustained-release capsules, which is used in bone diseases, pharmaceutical formulations, medical preparations with inactive ingredients, etc. Short intervals and other problems, to achieve the effect of being suitable for large-scale production applications, stable product quality, and reducing dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] A preparation method for alfacalcidol sustained-release capsules, comprising the following steps:
[0022] (1) Get a certain amount of microcrystalline cellulose powder and put it in a centrifugal granulator, and use water as a binder to prepare a blank pellet core with a particle size of 50 mesh;
[0023] (2) take alfacalcidol, filler and binder by weight percentage, binder is made into 5% aqueous solution, alfacalcidol and filler are put in the powder supply chamber of centrifugal granulator , take an appropriate amount of the blank pellet core obtained in step (1) and put it in a granulation pot, use the aqueous solution of the binder as the binder to prepare drug-containing pellets, dry the pellets at 60°C after they are taken out of the pot, and sieve them into 20 meshes. Next step coating;
[0024] (3) Dissolving the slow-release material, plasticizer, porogen, and anti-tack agent with 80% ethanol solution to make a slow-release coating solution;
[0025] (4) Ev...
Embodiment 1~3
[0027] The preparation of embodiment 1~3 alfacalcidol sustained release capsule
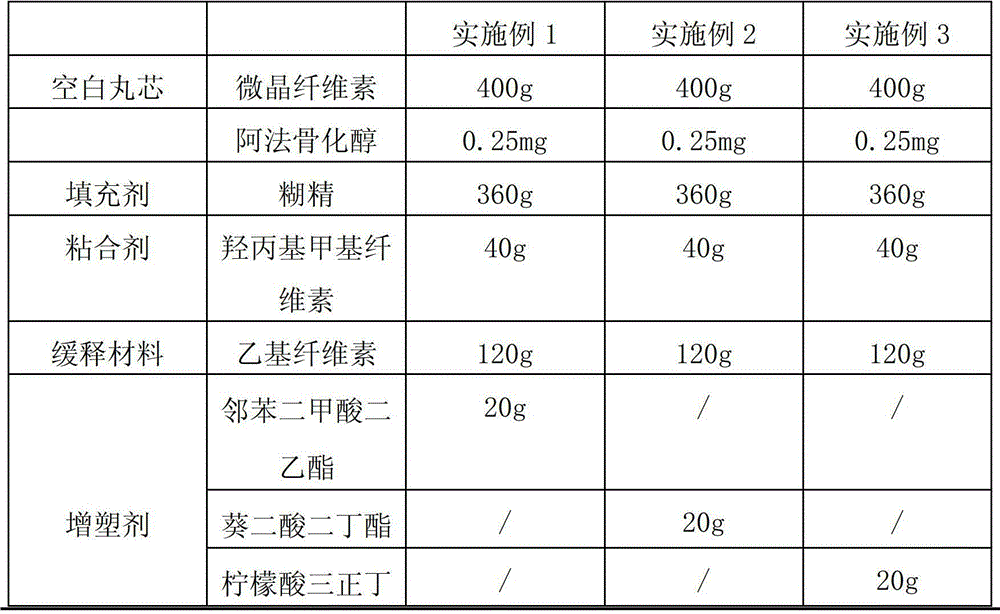
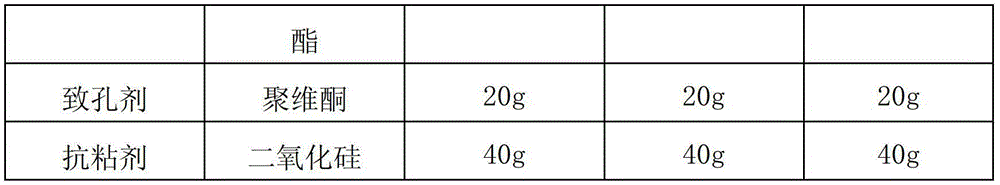
[0028] According to the raw and auxiliary materials in the following table, according to the above-mentioned preparation method, 2000 alfacalcidol sustained-release capsules were respectively prepared in each embodiment. Among them, " / " means not used.
[0029]
[0030]
[0031] Test Example 1 Determination of Release of Alfacalcidol Sustained-release Capsules Gained in Examples 1-3
[0032] According to the "Guidelines for Sustained and Controlled Release Preparations" in the appendix of the 2010 edition of the Pharmacopoeia of the People's Republic of China (Part II), 0.25% sodium lauryl sulfate was used as the release medium, and the preparations in Examples 1 to 3 were accurately weighed. An appropriate amount of alfacalcidol sustained-release pellets (about 100 mg) was determined according to the first method of appendix XD of the 2010 edition of the Pharmacopoeia of the People's Repu...
Embodiment 4~6
[0036] The preparation of embodiment 4~6 alfacalcidol sustained-release capsules
[0037] According to the raw and auxiliary materials in the following table, according to the above-mentioned preparation method, 2000 alfacalcidol sustained-release capsules were respectively prepared in each embodiment. The weight ratio of the slow-release material of embodiment 4 and the plasticizer is 8:1, the weight ratio of the slow-release material of embodiment 5 and the plasticizer is 7:1, and the weight ratio of the slow-release material of embodiment 6 and the plasticizer The weight ratio is 6:1.
[0038]
[0039] plasticizer
[0040] Test example 2 Determination of the release rate of alfacalcidol sustained-release capsules obtained in Examples 4-6
[0041] The measuring method is the same as that of Test Example 1. The measurement results are shown in Table 2.
[0042] Table 2 Example 4-6 Alfacalcidol Sustained-release Capsules Release Evaluation Form (Dissol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com