Alfacalcidol derivative and preparation method thereof
A technology for alfacalcidol and its derivatives, which is applied in the field of alfacalcidol derivatives and their preparation, can solve problems such as poor solubility and dissolution, obstacles to clinical promotion of drugs, and low solubility, and achieve improved water solubility , The effect that is conducive to clinical use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
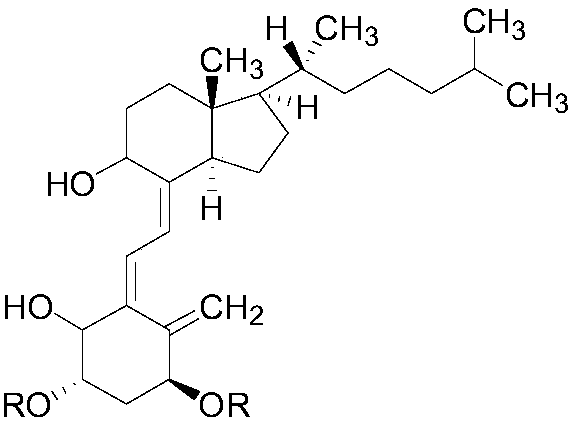
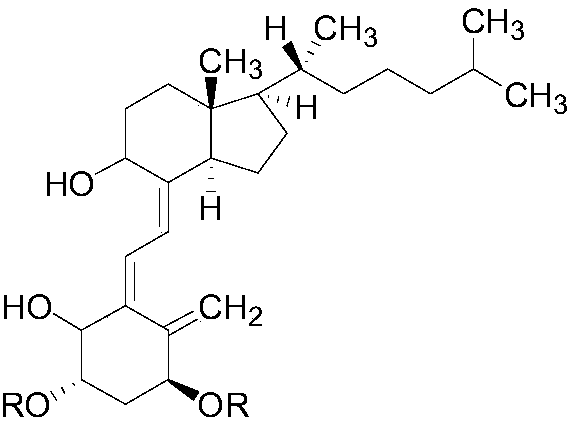
[0020] This embodiment discloses a class of alfacalcidol derivatives, the molecular structural formula of which is:
[0021]
[0022] R selected from
Embodiment 2
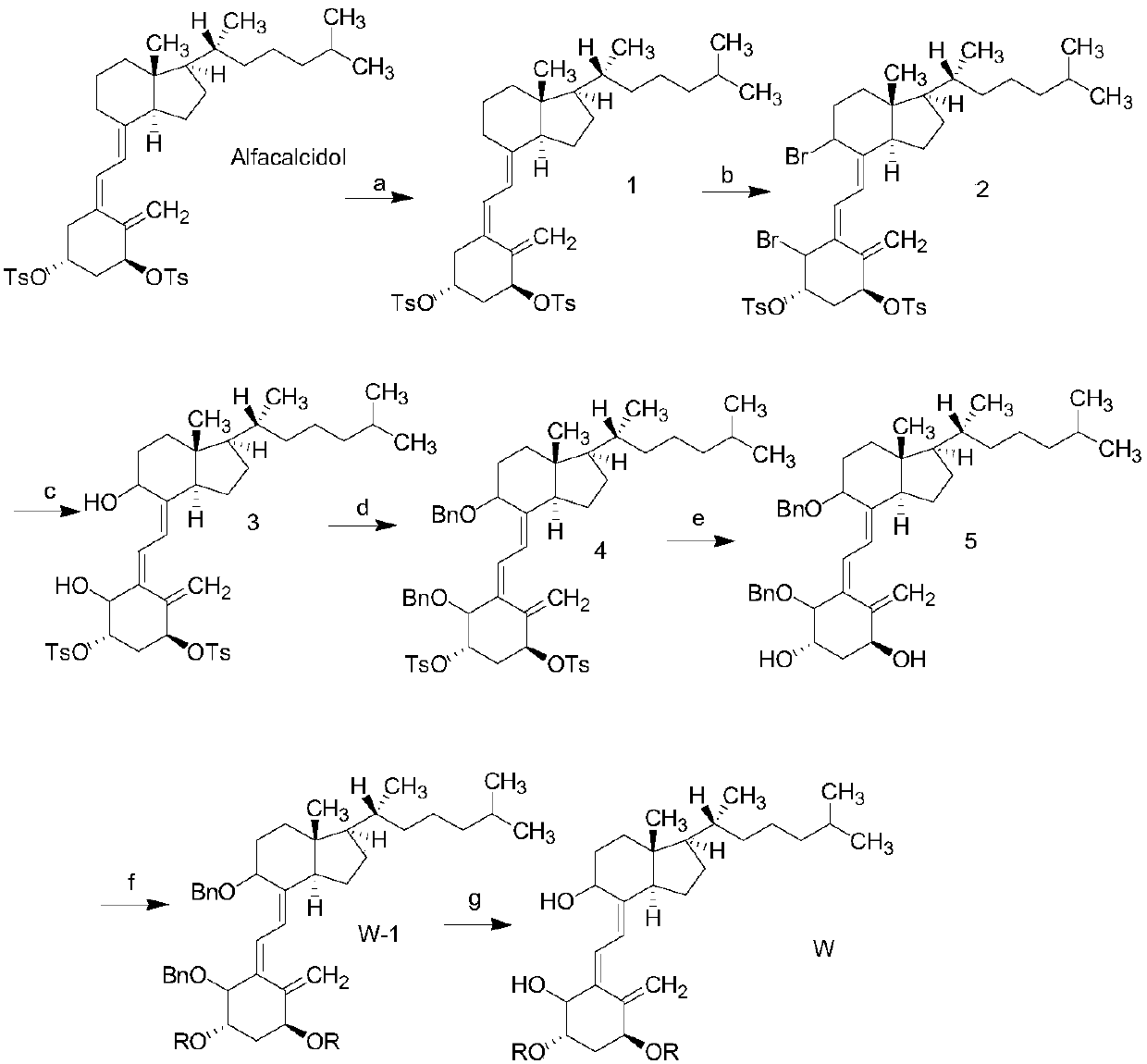
[0024] This embodiment discloses a preparation method of a class of alfacalcidol derivatives, the chemical equation is:
[0025]
[0026] The preparation method of intermediate 1 is as follows: add 100ml of dichloromethane, 2ml of pyridine, 4.12g of alfacalcidol, 4g of p-toluenesulfonyl chloride into a 250ml reaction bottle, put in an ice bath, stir the reaction overnight with magnetic force, extract the reaction with 5ml of ice water, pump After filtration, the filtrate was concentrated, and the residue was recrystallized from ethanol to obtain 5.9 g of white solid.
[0027] The molecular structural formula of intermediate 1 is:
[0028]
[0029] The preparation method of intermediate 2 is as follows: add 50ml of dimethyl sulfoxide, 7.22g of intermediate 1, and 2.5g of NBS to a 100ml reaction bottle, put it in an ice bath, react under light conditions for 4 hours, add 50ml of ethyl acetate, and extract the mixed solution with ice water for 3 times. The organic layer wa...
Embodiment 3
[0048] This embodiment discloses a preparation method of a class of alfacalcidol derivatives, the chemical equation is:
[0049]
[0050] The preparation method of intermediate 1 is as follows: add 100ml of dichloromethane, 2ml of pyridine, 4.12g of alfacalcidol, 4g of p-toluenesulfonyl chloride into a 250ml reaction bottle, put in an ice bath, stir the reaction overnight with magnetic force, extract the reaction with 5ml of ice water, pump After filtration, the filtrate was concentrated, and the residue was recrystallized from ethanol to obtain 5.9 g of white solid.
[0051] The molecular structural formula of intermediate 1 is:
[0052]
[0053] The preparation method of intermediate 2 is as follows: add 50ml of dimethyl sulfoxide, 7.22g of intermediate 1, and 2.5g of NBS to a 100ml reaction bottle, put it in an ice bath, react under light conditions for 4 hours, add 50ml of ethyl acetate, and extract the mixed solution with ice water for 3 times. The organic layer wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com