Method for green synthesis of 1,2-diamine compound under catalysis of visible light
A technology for green synthesis and aldehyde compounds, which is applied in the preparation of organic compounds, chemical instruments and methods, and preparation of aminohydroxy compounds, etc., can solve the problems of unobtainable raw materials, high requirements for reaction temperature and pressure, and long reaction time, etc. Achieving the effect of good yield, mild reaction conditions and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
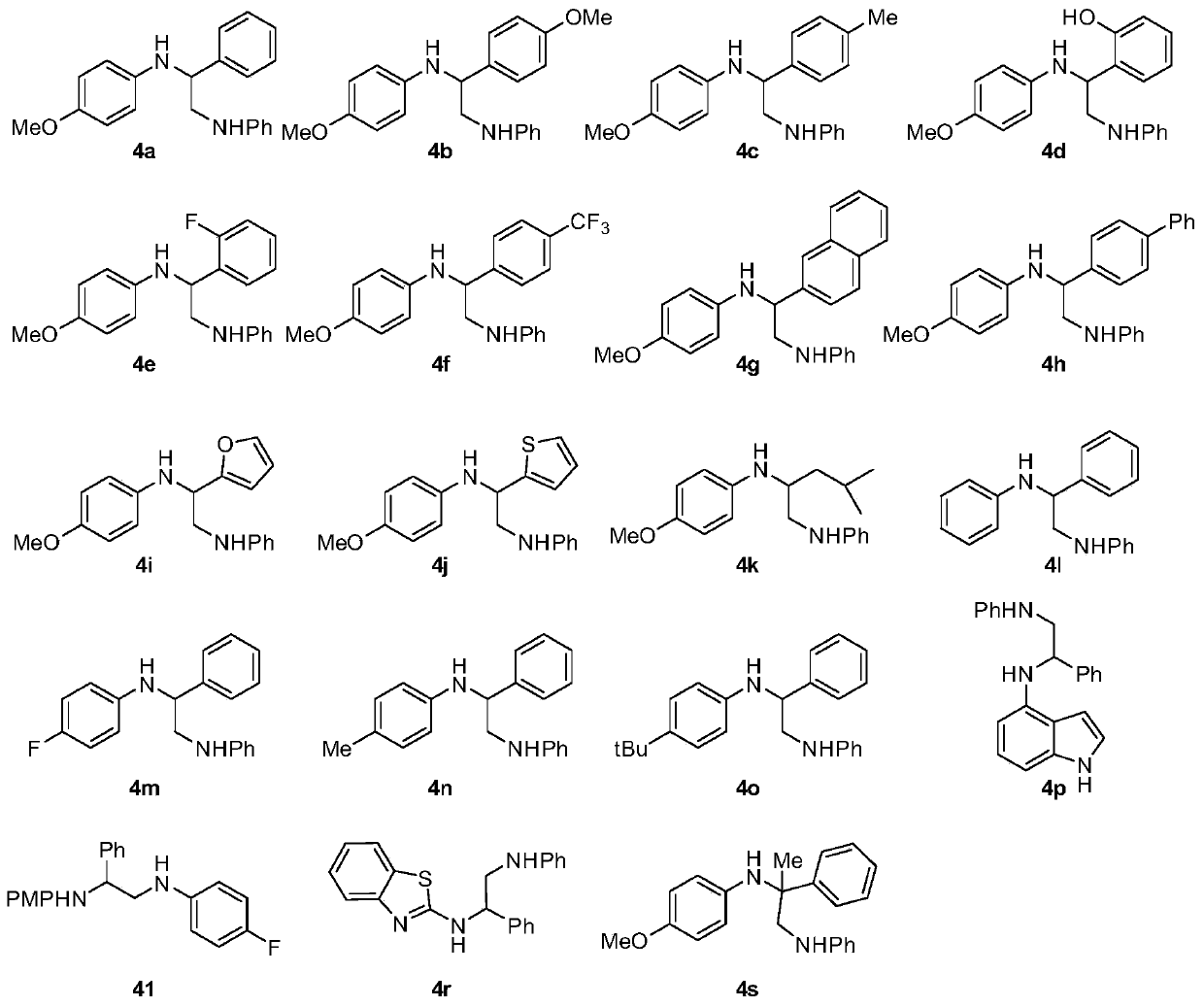
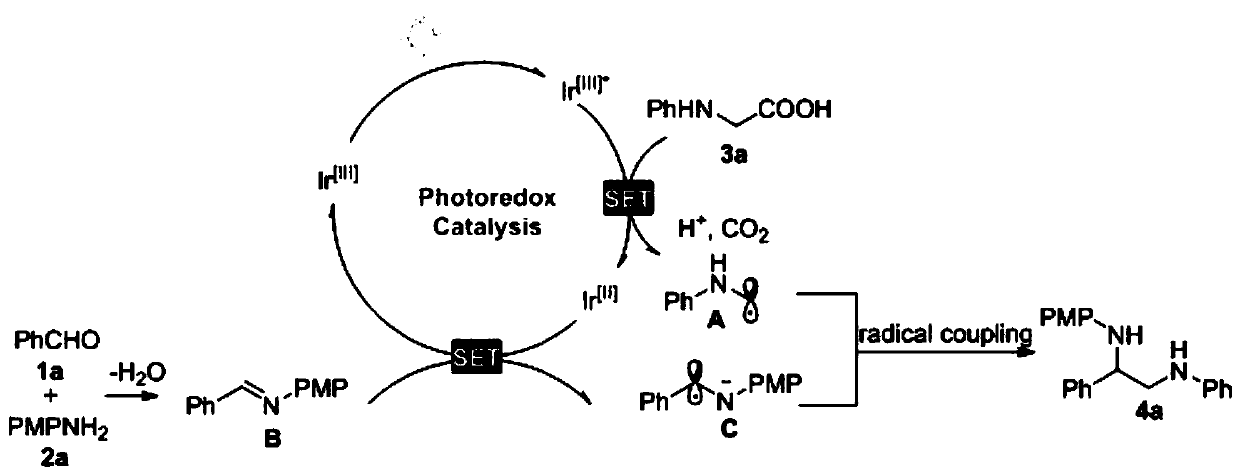
[0075] (1) Add 90.4 mg N-phenylglycine, 24.7 mg p-methoxyaniline, 2.6 mg photocatalyst {Ir[dF(CF 3 )ppy] 2 bpy}PF 6 , 2 mL 0.1M N , N - Dimethylacetamide, 21.2 mg benzaldehyde, the ratio of benzaldehyde: p-methoxyaniline: N-phenylglycine: photocatalyst is 1:1:3:0.01.
[0076] (2) The Schlenk tube obtained in step (1) is pumped and ventilated three times to ensure that there is no water and oxygen in the reaction tube before sealing it.
[0077] (3) Place the Schlenk tube obtained in step (2) under the irradiation of a Blue LED with a lamp source of 10W and a lambda of 455 nm, and carry out stirring reaction at room temperature for 2 hours. The distance between the Blue LED lamp and the Schlenk tube is 3cm. When TLC detects that the aldehyde compound disappears, the reaction is terminated, and the developer ratio of the TLC detection reaction is PE:EA=10:1.
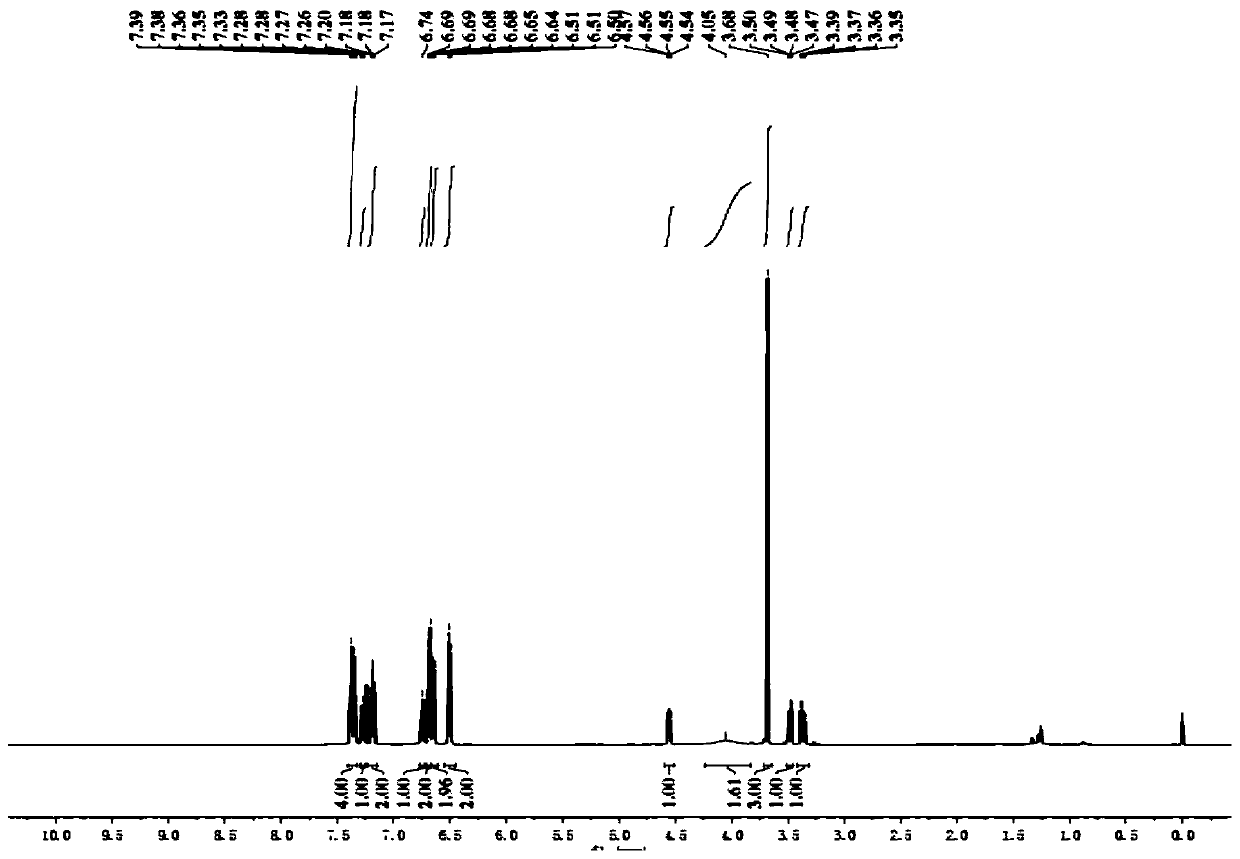
[0078] (4) Add 15 mL of water to the Schlenk tube obtained in step (3) to quench the reaction, transfer to a separa...
Embodiment 2
[0082] (1) Add 90.4 mg N-phenylglycine, 24.7 mg p-methoxyaniline, 2.6 mg photocatalyst {Ir[dF(CF 3 )ppy] 2 bpy}PF 6 , 2 mL N , N -Dimethylacetamide, 27.2 mg p-methoxybenzaldehyde, the ratio of p-methoxybenzaldehyde: p-methoxyaniline: N-phenylglycine: photocatalyst is 1:1:3:0.01.
[0083] (2) The Schlenk tube obtained in step (1) is pumped and ventilated three times to ensure that there is no water and oxygen in the reaction tube before sealing it.
[0084] (3) Place the Schlenk tube obtained in step (2) under the irradiation of a Blue LED with a lamp source of 10W and a lambda of 455 nm, and carry out stirring reaction at room temperature for 2 hours. The distance between the Blue LED lamp and the Schlenk tube is 3cm. When TLC detects that the aldehyde compound disappears, the reaction is terminated, and the proportioning of the developer during the TLC detection reaction is PE:EA=10:1.
[0085] (4) Add 15 mL of water to the Schlenk tube obtained in step (3) to quench the...
Embodiment 3
[0087] (1) Add 90.4 mg N-phenylglycine, 24.7 mg p-methoxyaniline, 2.6 mg photocatalyst {Ir[dF(CF 3 )ppy] 2 bpy}PF 6 , 2 mL N , N -Dimethylacetamide, 24.1 mg p-tolualdehyde, the ratio of p-tolualdehyde: p-methoxyaniline: N-phenylglycine: photocatalyst is 1:1:3:0.01.
[0088] (2) The Schlenk tube obtained in step (1) is pumped and ventilated three times to ensure that there is no water and oxygen in the reaction tube before sealing it.
[0089] (3) Place the Schlenk tube obtained in step (2) under the irradiation of a Blue LED with a lamp source of 10W and a lambda of 455 nm, and carry out stirring reaction at room temperature for 2 hours. The distance between the Blue LED lamp and the Schlenk tube is 3cm. When TLC detects that the aldehyde compound disappears, the reaction is terminated, and the proportioning of the developer during the TLC detection reaction is PE:EA=10:1.
[0090] (4) Add 15 mL of water to the Schlenk tube obtained in step (3) to quench the reaction, tra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com